Enzymes-rate of reaction
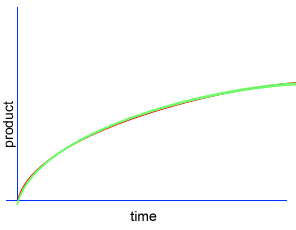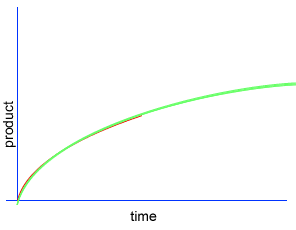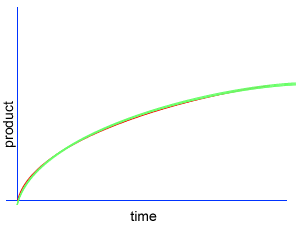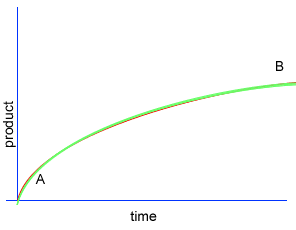
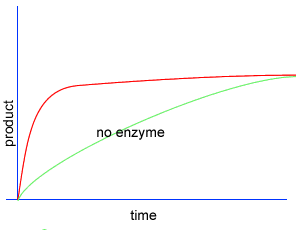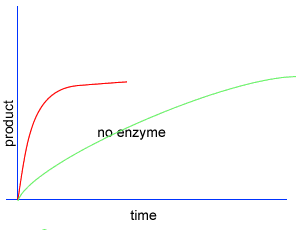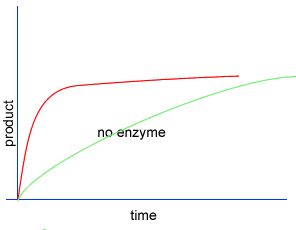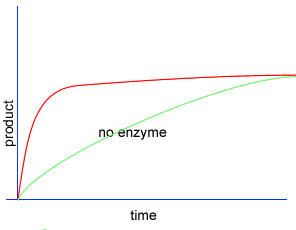
A chemical reaction proceeds when molecules with sufficient kinetic energy collide, break apart and recombine into a new chemical compound.
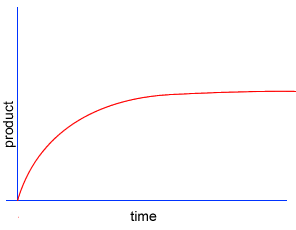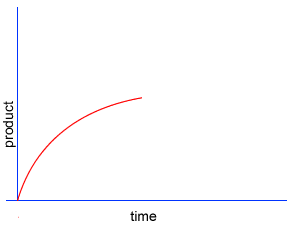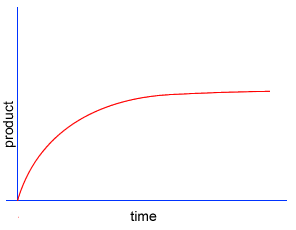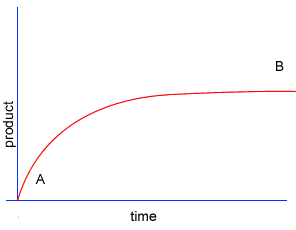
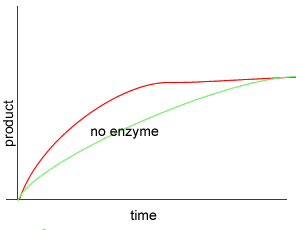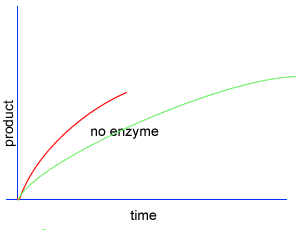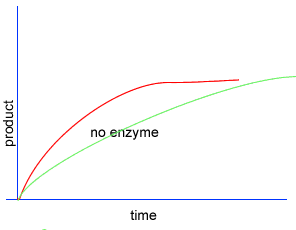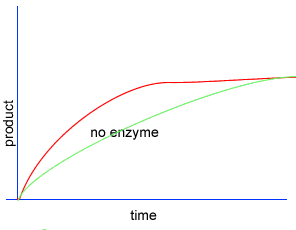
Initially product is formed at a higher rate, as shown by section A on the graph on the left, as there are plenty of substrate (reactant) molecules about. As substrate molecules become more scarce, the rate of product formation slows down, as shown in section B of the graph on the left.