Immune system- antibodies
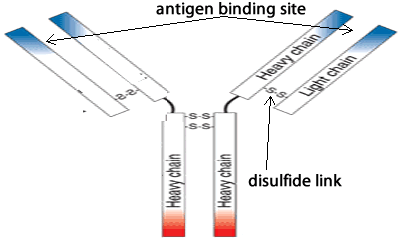
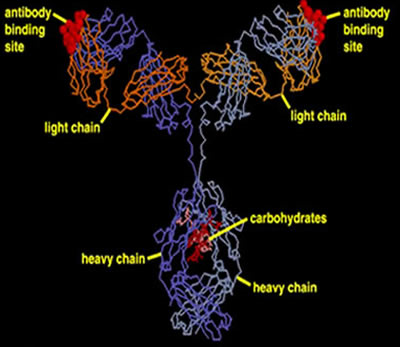
An antibody, also known as an immunoglobulin, is a large Y-shaped molecular structure largely composed of protein. Antibodies form part of the immune system and are used to identify and attach to foreign objects such as bacteria and viruses. As shown on the right, an antibody is made of heavy protein chains and two light. The chains are connected by covalent bonds between sulfur atoms called disulfide links.
The"Y" tips of the antibody, shown in blue, have specific recognition of unique parts of pathogens or cells identified for destruction, termed antigens. Each tip of the "Y" of an antibody contains the same specificity for the particular antigen.
Antibodies can neutralize targets directly, by blocking a part of a microbe that is essential for its invasion and survival. The production of antibodies is the main function of the humoral immune system.
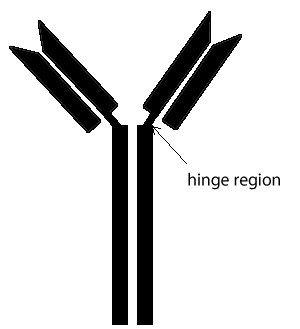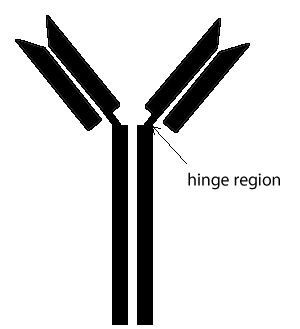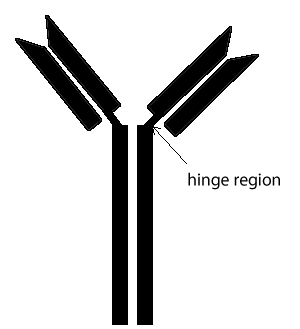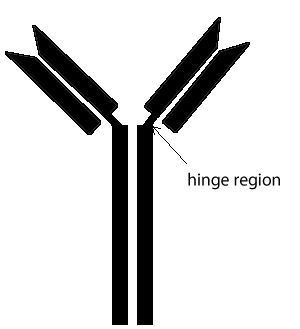
The region between the arms and the body of the Y structure, known as the hinge region, permits flexibility between the two arms of the Y-shaped antibody molecule. This allows them to open and close to accommodate binding to two identical antigens separated by a fixed distance.

View the video on the right and answer the questions below.
The chemistry of the antibody.
Explain why antibodies fall into the class of molecules called glycoproteins?
The difference from one antibody to another is the
How are the protein chains held together to form the antibody?
Using the term "hypervariable loops" discuss how antibodies have such specificity for antigens.