Water interactions intermolecular bonding exercises.
Ethanol has the structural formula CH3CH2OH . Ethanol is placed in coloured water.
What do you think will happen when the tube is shaken?
Explain why?

Consider the molecule of acetone shown on the right.
Is it a polar molecule?
Does it display hydrogen bonding? Explain.
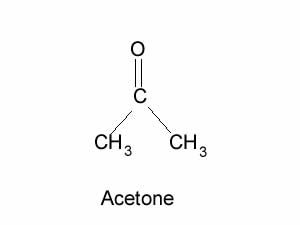
Acetone was placed in coloured water as shown on the right. Do you think it will dissolve in water? Click to see.
What can you say about the polar nature of the acetone molecule?

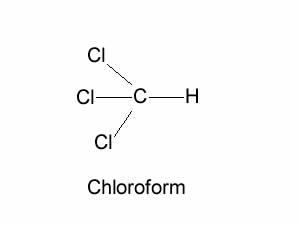
Consider the chloroform molecule shown on the right.
Is this a polar or non-polar molecule?
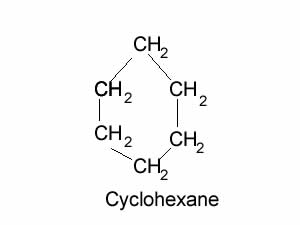
Do you expect cyclohexane to dissolve in water? Explain
Cyclohexane is placed in water. It is left for a few minutes as shown on the right.
Explain why water and cyclohexane are immiscible?
What is the main intermolecular force of attraction in cyclohexane and water.
Which is more dense? Can you explain.

Chloroform is shown on the right.
Is it a polar molecule?
Will it dissolve in water?
Cyclohexane is placed in water and left for a few minutes, as shown on the right.
Explain why chloroform, being a polar molecule, is not soluble in water?

The substances water, chloroform and cyclohexane were mixed together and left for a few minutes as shown on the right.
Water is the smallest molecule out of the three with a molecular mass of 18. Cyclohexane is the next largest molecule with a molecular mass of 84 and chloroform the largest with a molecular mass of close to 119. You would expect that water would float on top of cyclohexane with chloroform at the bottom, as is the case with the latter. Give a possible explanation.
