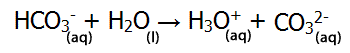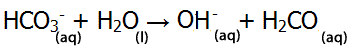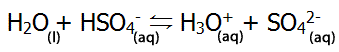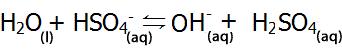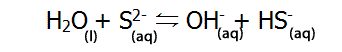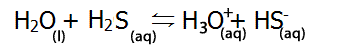|
An amphiprotic substance is one that can either donate a proton (act as an acid) or accept a proton (act as a base)
Below are some examples of amphiprotic substances. |
| HCO3- can act as an acid (donating a proton), as shown on the right. |
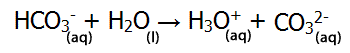 |
| Or HCO3- can act as a base (accepting a proton) |
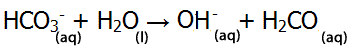 |
| HSO4- can act as an acid (donating a proton), as shown on the right |
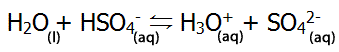 |
| Or HSO4- can act as a base (accepting a proton) |
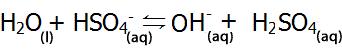 |
| H2O can act as an acid (donating a proton) |
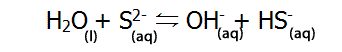 |
| Or H2O can act as a base (accepting a proton) |
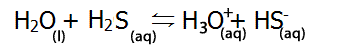 |
Write the reactions of HCO3- with OH- and HCO3- with H3O+
Solution |
 |
Write the reactions of HSO4- with OH- and HSO4- with H3O+
Solution |
Write the reactions of H2PO4- with OH- and H2PO4- with H3O+
Solution |