Before we can measure the concentration
of an element, in this case lead, we must first graph the absorbance of
known concentrations of the element. This is shown below.
The absorbance of known solutions were
| Absorbance |
2
|
4
|
6
|
8
|
10
|
| Concentration of lead in ppm |
5
|
10
|
15
|
20
|
25
|
Once the absorbance of known solutions is graphed we can measure the absorbance of an unknown sample and using the graph, read the concentration, as shown below.
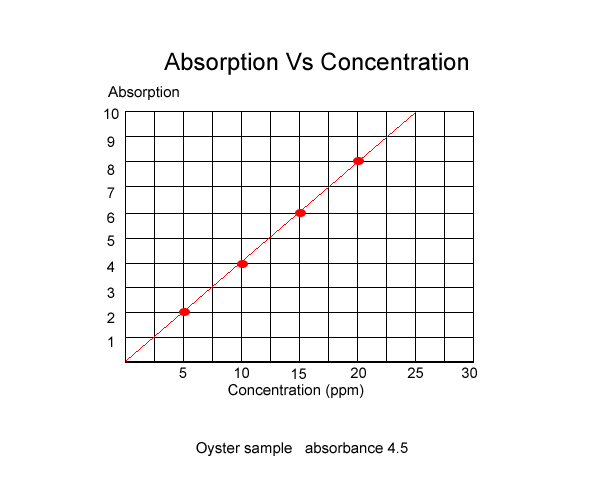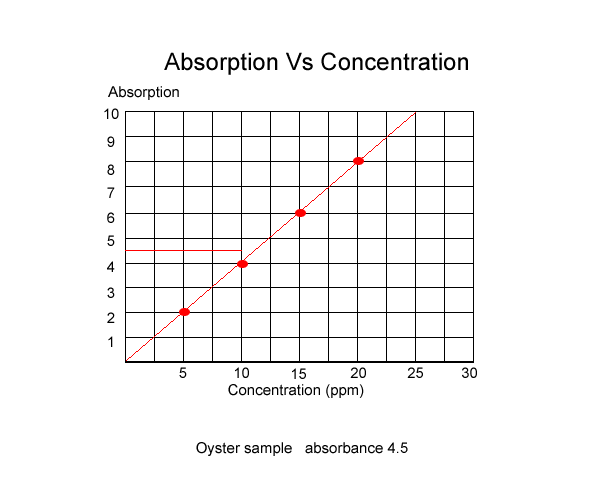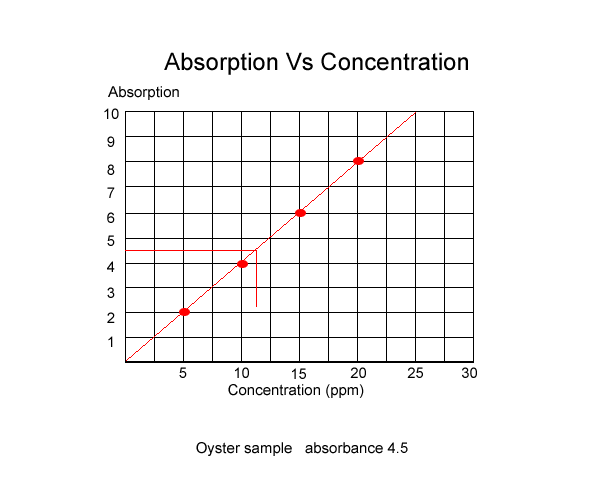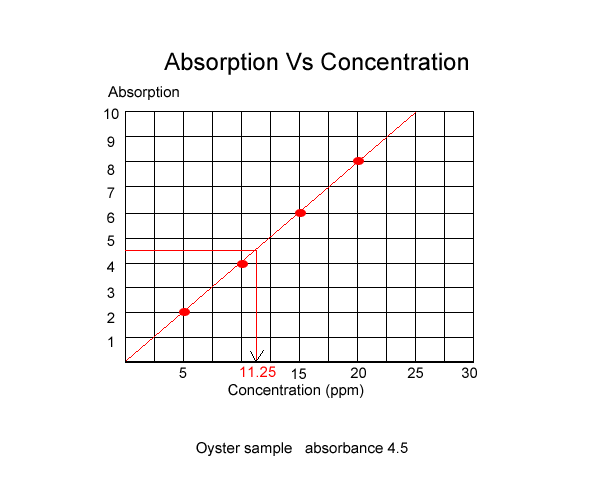
A sample of oyster was tested for lead content using atomic absorption spectroscopy and was found to have a lead concentration of 11.25 ppm.
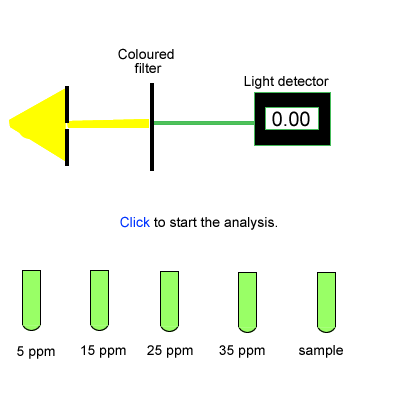
Construct an absorption vs concentration graph.
What is the lead concentration
of the 1 ml sample?
What is the lead content present in the 2.00 gram of oyster sampled?
What is the lead concentration in the oyster per gram of oyster flesh?
If the recommended limit of lead in oysters for human consumption is 3.00 ppm in every gram, can you recommend the oysters for sale?