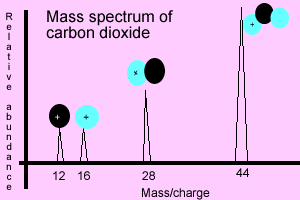
Mass spectrum of
carbon dioxide
The animation below shows how the carbon dioxide molecule is ionised in a mass spectrometer. Under extreme heat the molecule decomposes into distinct charged fragments (ions). The molecule will always shatter into the same fragments when subjected to the right amount of heat. Every molecule shatters in its own unique way and therefore mass spectrometry can be used to identify chemical substances present in unknown samples. The mass spectrum is the molecule's unique chemical fingerprint, the bigger the molecule the more complex the spectrum becomes.
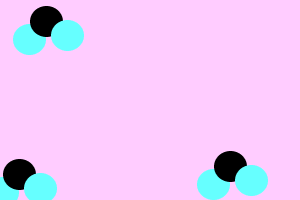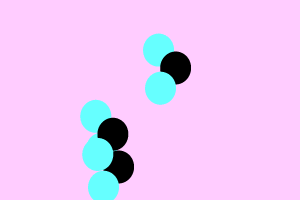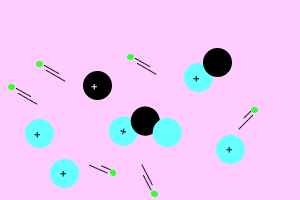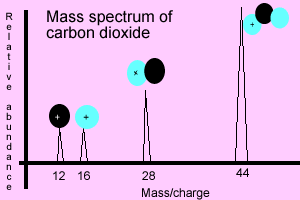
Carbon dioxide is released into the ionising chamber where the heat is enough to shatter the molecule into different fragments. Electrons absorb the energy (heat) and fly off the fragments leaving behind positive charged fragments (ions). Scan the image on the right to reveal the unique fragments that the carbon dioxide molecule shatters into.
Each
charged fragment will produce its own peak in the spectrum.