Below is a table of some typical covalent bonds and their characteristic wavenumber at which energy is absorbed.
Bond
type |
Type
of molecule found in. |
Wavenumber |
C-O |
Alcohols
and esters |
1000-1300 |
C=O |
Carboxylic
acids and esters |
1680-1750 |
C=C |
Alkenes |
1600-1700 |
C-H |
Alkanes |
2850-3100 |
C-H |
Alkenes |
3000-3100 |
O-H |
Carboxylic
acids |
2500-3300 |
O-H
|
Alcohols |
3200-3600 |
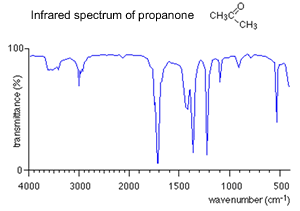
C-H trough is
seen at about 3000 .
C=O double bond is seen at about 1740 .
C-O single bond is seen at about 1240.

As you can see the C=O group at a wavenumber of about 1700 is prominent in both as is the C-H group at about 3000. However this is a good example of how you should take care as IR spectra of different groups of compounds can, at first, look very similar. You will find that this is very similar to the infra-red spectrum for ethyl ethanoate, an ester.
However, there are troughs which look as if they might be due to C-O single bonds which are not present in propanone.