The smallest particle of matter is called an atom. The atom consists mostly of space. If you were to take the atom as being the size of the MCG (Melbourne Cricket Ground), then the nucleus (the centre of the atom) is the size of a flea in the middle of the ground while buzzing around the outside of the stadium are the electrons a thousand times smaller than a flea. The atom is made up of even smaller sub-atomic particles called neutrons, protons and electrons. It has a nucleus made of protons and neutrons and around this nucleus revolve the electrons with a great deal of energy. Although electrons have a great deal of energy the nucleus has a phenomenal amount of energy. The energy during a nuclear explosion actually comes from an atom's nucleus. |
|
| There are
just over 112 different types of atoms. We call the different types
of atoms elements. The first ten elements are 1 hydrogen (H) 2 helium (He) 3 lithium(Li) 4 beryllium (Be) 5 boron(B) 6 carbon(C) 7 nitrogen(N) 8 oxygen(O) 9 fluorine(F) 10 neon(Ne). |
The energy from the nucleus is released during nuclear explosions. |
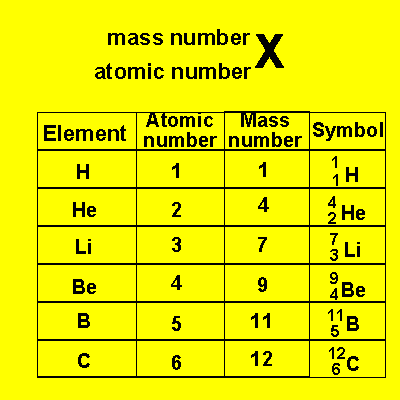
The number of the element has special significance. It is called the atomic number. The atomic number identifies the element, that is to say, all elements have a different atomic number. The atomic number tells us how many protons are present in all the atoms of a particular element. For example carbon has an atomic number of 6. All carbon atoms have 6 protons in their nucleus. The atomic mass of an atom is the number of protons and neutrons added together. For example, an atom with 6 protons and 4 neutrons is a carbon atom with an atomic mass of 10 (6+4).
We have a special way of writing
the elements with their symbol, mass number and atomic number. See below.