The atom
Fluorine gaining electrons
Fluorine needs one electron to fill the outer energy level. It will get one electron from another atom.
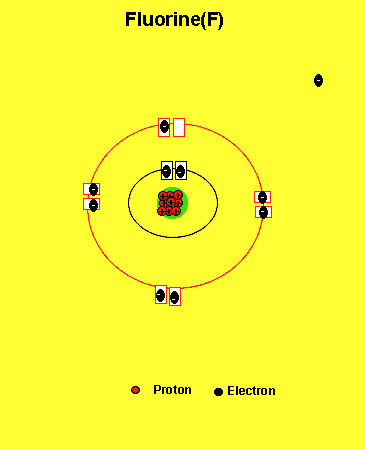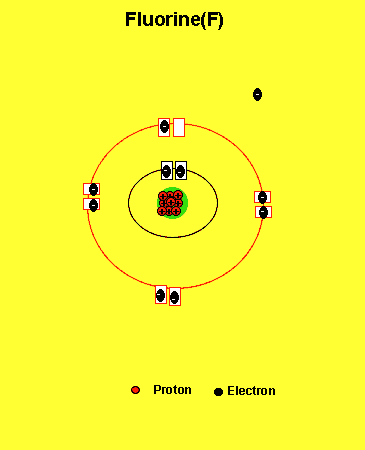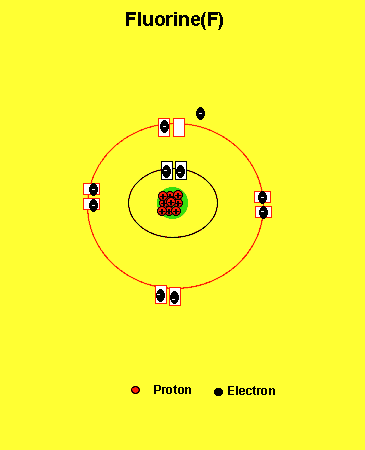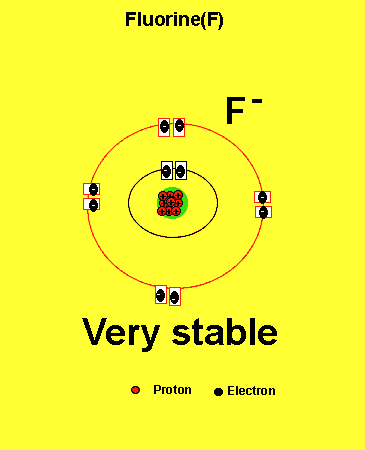
Notice how the fluorine atom takes one electron to look like neon. We can tell that it is afluorine atom by the number of protons in its nucleus. The imbalance of protons and electrons now creates a charge of -1 on the atom.
Atoms with a charge are known as IONS. More precisely atoms with a positive charge are known as cations while atoms with a negative charge are known as anions. Fluorine, above, forms an anion.