Atoms and their electrons
It is hard to explain why atoms react with each other and produce energy in chemical reactions. One very successful way of introducing the concept of atoms and electrons in chemical reactions is outlined below. It attempts to give students an understanding as to how and why atoms transfer electrons from each other in chemical reactions.
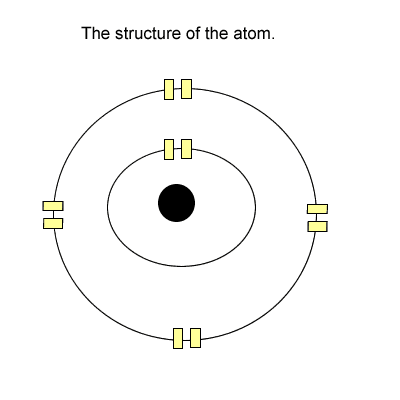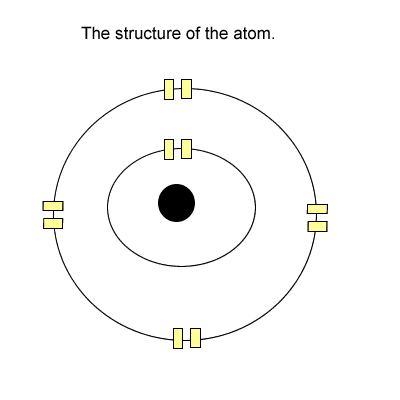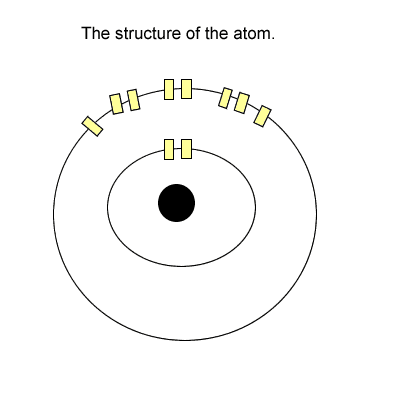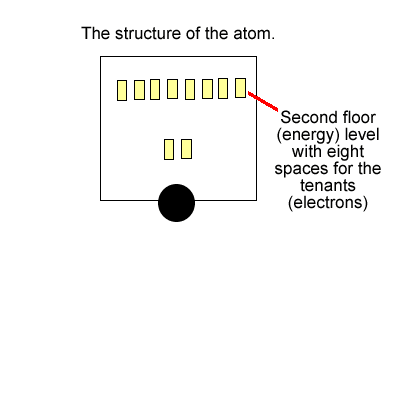
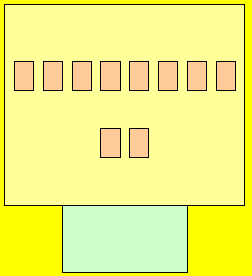
The tenants (electrons) stay in these apartments. Now each level has one light switch. If there is one or more tenant(electron) on any level the entire level is energised with light.
Atoms are very strange little things. They have this unique habit of always wanting to be 100% energy efficient.
Lets see what we mean by 100% energy
efficient.
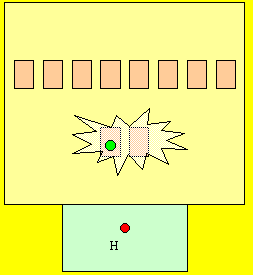
Take hydrogen for example, it has one electron in the first level. The entire first level is energised. Is this 100% energy efficient? Click to see how hydrogen reacts with oxygen to exchange eelctrons.
NO! Why?
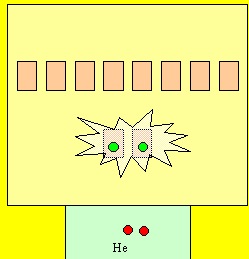
Now take helium, it has two electrons
in the first level. The entire first level
is energised. Is this 100% energy
efficient?
YES! Why?