The atom
what is it?
The atom is said to be the smallest
particle of matter. But what is it?
It is a small spherical object made of subatomic particles called protons,
neutrons and electrons. Protons are positively charged, electrons have a
negative charge while neutrons have no charge. The volume of the atom is mostly space, with most of its mass concentrated in a small region in the middle of the atom called the nucleus.
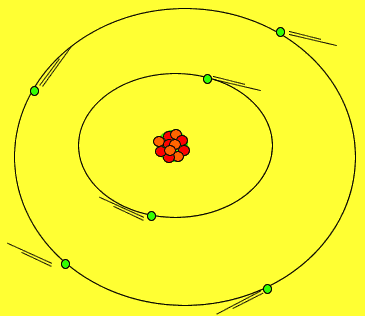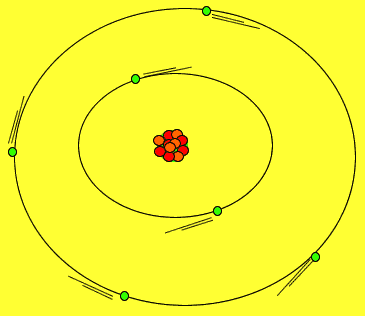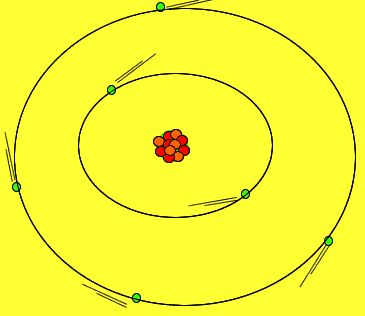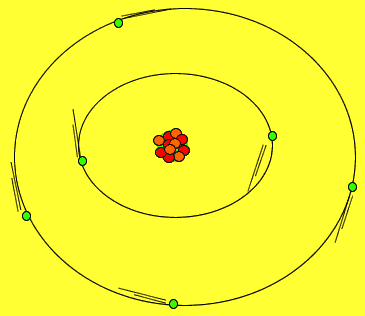
Protons and neutrons are tightly packed in a small solid sphere called the nucleus of the atom. Around this nucleus is a cloud of electrons. Electrons move about in random motion at different levels around the nucleus depending on their energy. The greater the energy of the electron the further away from the nucleus the electron can be found.
The image on the right show a hydrogen atom and the electron cloud formed by a single electron traveling around the nucleus.
Click to see the helium atom
Click to see the carbon atom
Click to see the oxygen atom
Click to see the gold atom

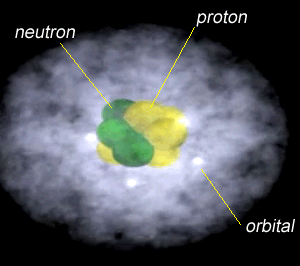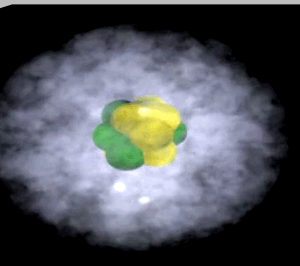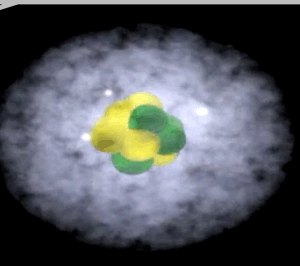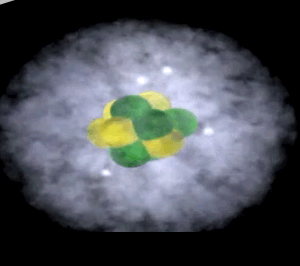
The image on the right shows the nucleus of a carbon atom with electrons revolving around it. The size of the nucleus is greatly exaggerated.
Notice how the protons are tightly packed in the nucleus. Because protons have a positive charge they should be repelling each other. However a very strong force, called the strong nuclear force, binds them tightly in the nucleus.
Electrons are held close to the nucleus by electrostatic forces of attraction, positive protons attract the negative electrons.
As shown in the animation on the right, electrons orbit the nucleus in special regions of space surrounding the nucleus called orbitals.
To put you in the picture imagine the atom the size of the MCG (Melbourne Cricket Ground). The nucleus would be a ping-pong ball in the centre wicket and the electrons would be the size of a flea orbiting around the outside of the ground. All in all, the atom is made up of empty space.

The atom contains a great deal of energy and we make good
use of all its energy. Its electrons contain energy which is released
during chemical reactions that fuel our fires and our vehicles.
Its nucleus contains an even greater amount of energy, which can be released with devastating consequences. Nuclear
explosions are examples of the energy contained within the nucleus.

For nuclear fusion reaction to occur a great deal of energy is required. Since the protons are positively charged suggest what happens when two protons come together?
Why is a great deal of heat energy required to bring protons together
and create a bigger atom in fusion reaction?
The volume of the atom is mostly made up of
The nucleus of the atom is composed of
What is the charge on an electron
What is the charge on an proton
What is the charge on an neutron