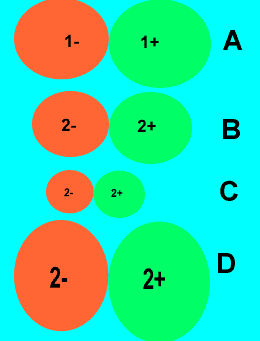
|
Look at the ionic compounds
on the right. The relative size and charge of each ion is shown.
From what you know of the properties of ionic compounds answer the
following questions.
1)Place the compounds
in order of increasing melting point.
The strength of electrostatic forces is directly related to size of charge and inversely proportional to distance between the charged particles.
eg. the bigger and closer the charges are the greater the electrostatic force of attraction or repulsion will be.
Hence in order of increasing MP
A => D => C => B where B has the highest MP.
2)Which compound conducts
electricity in the solid and molten states?
All of the ionic compounds will conduct electricity ONLY in the liquid state. So none of the compounds will conduct electricity in the solid state.
3) Which coloured ion
(green or orange ) is the metal ion?
Green, as all metal atoms will donate electrons to non-metal atoms to form positive ions (cations).
|
|
|
Atom
|
Electronic
configuration
|
|
X
|
2,8,2
|
|
Y
|
2,8,1
|
|
Z
|
2,8,6
|
|
D
|
2,8,3
|
|
R
|
2,7
|
|
O
|
2,6
|
Above is the electronic
configuration of some elements. Answer the following questions.
1) Which one of the following
compounds will have the highest melting temperature? Explain.
The compound
formed from element X and element O
or the compound formed from element Y and element R .
Compound formed between X and O. The charged ions are 2+ and 2- respectively whilst the charged particles between the Y and R are 1+ and 1- respectively. Since the distance between each pair of ions is relatively the same the charges will figure more prominently in determining the strength of the electrostatic force of attraction.
2) Answer True of False to the following comments.
a) The ion
formed by element "X is bigger than the ion formed by element
"Y". False - element X will form X2+ whilst element y will form Y1+ A 2+ charge will attract the 8 valence electrons more strongly than a 1+ charge, hence the 8 valence electron of X2+ will experience a greater force of attraction than the 8 valance electron of Y1+.
b) The ion formed by element "Z" will have a charge of 2+. False -It is anon-metal and will except 2 electrons from metal atoms or atom to form an anion with a charge of 2-.
c) An ionic compound can form between the elements "Z"
and "O" False -Ionic compounds form between two elements if one is a metal and the other is a non-metal. Both Z and O are non-metals hence they will share electrons and will form a covalently bonded molecule.
d) When element "Z" and element "X" react the
compound formed will be brittle and conduct electricity in the solid
state as well as the molten state. False -X and Z will form an ionic compound. Yes the compound will be brittle but will not conduct electricity in the solid state as the charges are not mobile as they are in the liquid state.
e) When elements "D" and "R" react "D"
will form the positive ion while "R" forms the negative
ion. True. D will give up 3 electrons to form the cation D3+ whilst R will accept one electron to form R1-
3) Jonathon was having problems conducting an electric current through
a sample of water. Stephen suggested dissolving a powder containing
a pure compound made form element "R" and "O".
Irene disagreed and suggested dissolving the soluble compound YR.
Who is right? Explain.
Irene is right. YR is an ionic compound that will dissolve to form Y1+ (aq) cations and R1- (aq) anions. Free flowing ions will conduct electricity in solution. A compound formed between two non-metals forms molecules rather than ions hence will not conduct electricity, to any great degree, when dissolved in water. |