A molecule is made up of non-metal atoms that share electrons with each other through the formation of covalent bonds. This sharing of electrons, however, is not always equal and the more electronegative atom takes a bigger share of the electrons than the less electronegative atom. For example, take the HF molecule, shown on the right, the more electronegative fluorine atom attracts the bonding electrons more strongly than the hydrogen atom. This uneven sharing of electrons causes small positive and negative charges to form on opposite ends of the molecule, known as dipoles. A molecule is said to be polar if one end of the molecule has a slight negative charge and the other a slight positive charge.

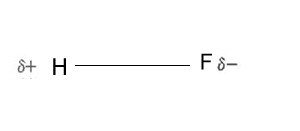
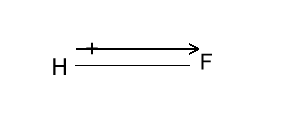
Deciding whether a molecule is polar or not depends on the type of bonds within the molecule and its shape.
There is one very useful rule to working out if a molecule is polar or not and it simply depends on the shape of the molecule.
All symmetrical molecules are non-polar and all asymmetrical molecules are polar.
Just remember this general rule
Symmetrical = non-polar molecule
Asymmetrical = polar molecule
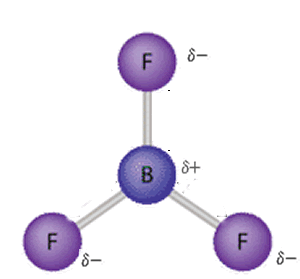
Although symmetrical molecules may have dipoles the dipoles cancel out due to the symmetrical nature of the molecule. Take BF3 for example, as shown on the right. It is a symmetrical molecule.
Each dipole is shown clearly, however, the symmetry of the molecule cancels out the dipole moments and results in a non-polar molecule. Click to see how the dipole moments cancel out.
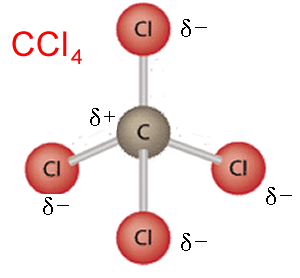
Take CO2 as another example, as shown on the right. It is also a symmetrical molecule. Each dipole is shown clearly, however, the symmetry of the molecule cancels out the dipole moments and results in a non-polar molecule. Click to see how the dipole moments cancel out.
NH3 , H2O, CH3Cl

Whether a molecule is polar or non-polar can determine the molecules physical properties. Polar molecules have permanent dipoles and tend to attract one another, creating permanent weak intermolecular bonds that hold the molecules together in the liquid and solid states. These forces determine the molecule's physical properties such as surface tension, melting and boiling temperatures and solubility in certain solvents. Polar molecules interact through intermolecular forces such as hydrogen bonding or dipole-dipole bonding.
Alternatively, non-polar molecules do not have these permanent charges, and so they have a weaker attraction or bonds with one another.

CH4 , CCl4 , or any molecule that is formed by a central carbon atom bonded to four identical atoms.
Cl2, O2, H2 etc.
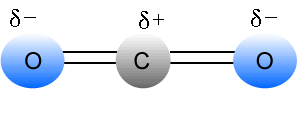
CO2, SO3, BF3.
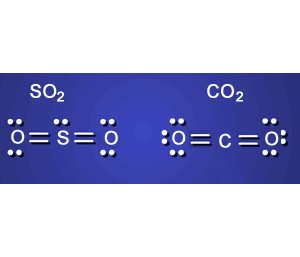
1) Consider the Lewis dot diagrams of the molecules CO2 and SO2 .
Which one is polar and which is non-polar?
Which molecule will dissolve fully in a polar solvent? Explain
Solution
2) Water and methane have roughly the same molecular mass and yet water is a liquid at room temperature while methane is a gas. Explain with reference to polarity of each molecule.
Solution

3) A diatomic molecule has a positive and negative end as shown on the right. Indicate whether the comments below are true or false.
a) This is a symmetrical molecule.
b) Atom A has a lower electronegativity than atom B
c) This molecule has the same molecular mass as CH4. Compared to CH4, this molecule is less soluble in water and more soluble in fat.
d) CH4. has a greater dipole moment than the molecule shown on the right.
4) Which of the following contributes to the molecule having a positive and a negative end?
i) Size of atoms
ii) Overall size of the molecule
iii) Electronegativity of both atoms
iv) Shape of the molecule.
Solution
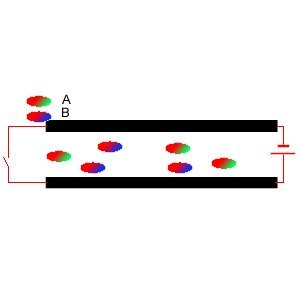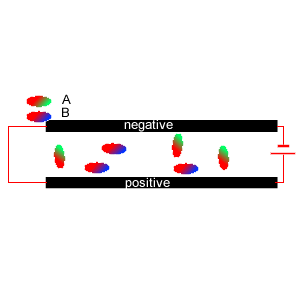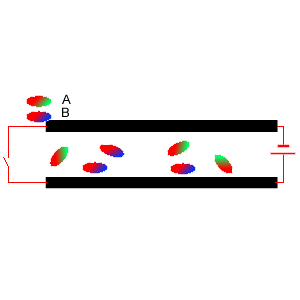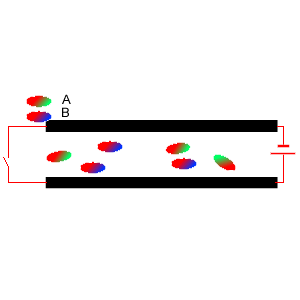
5) Two diatomic molecules A and B were mixed and placed between two charged plates, as shown on the right. Which comment is true?
i. Molecule A is symmetrical.
ii. Molecule B is asymmetrical
iii. It is likely that molecule B contains two atoms of the same element.
iv. Molecule A has the atom with the lowest electronegativity at its
green end.
Solution