The explosive chemistry
of nitrogen.
When we think of explosives, one element comes to mind time and time again. Nitrogen gas is a product of many explosive reactions. The compound nitroglycerin is pictured on the right. It is clear to see the importance of nitrogen in this compound.
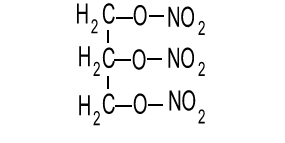
Nitrogen is a very stable molecule and has a very low energy state. For this reason the formation of nitrogen gas from a compound whose nitrogen atoms are bonded in a high energy state releases a great deal of energy. Consider the explosive decomposition of nitroglycerin according to the equation below.
4C3H5N3O9(l) => O2(g) + 6N2(g) + 12CO2(g) + 10H2O(g)
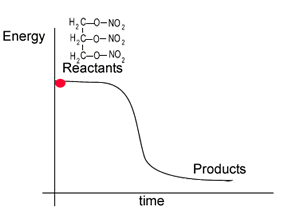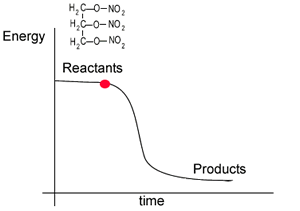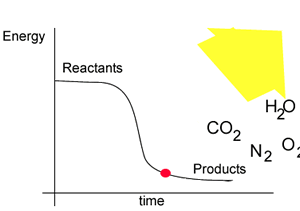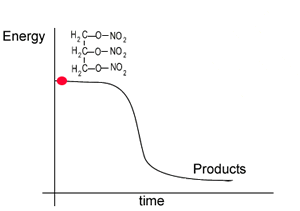
The energy profile for this reaction is shown on the right. Notice how the reactant, nitroglycerin, is at a higher energy state than the products. The difference in the energy of the reactants and products is given off as heat.
It is important for all explosive reactions to:
- be extremely exothermic
(release heat);
- produce gas. The gas produced expands violently with the heat produced
by the reaction.
- occur rapidly. The rapid nature of an explosive reaction is exemplified
by the comparison of the burning of 1 kg of petrol and 1 kg of T.N.T.
(trinitrotoluene). 1kg of petrol, if allowed to burn completely, produces
just over 30,000 kj, whereas the same mass of T.N.T. produces just over
4,000 kJ of energy. The explosive nature of T.N.T is due to the fact
that the energy is released in microseconds rather than seconds, as
in the case with petrol.

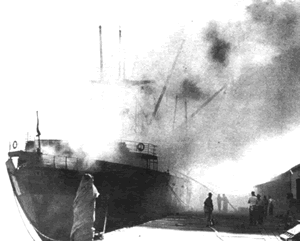
The 7,200 ton
ship was lifted several metres out of the water and the resulting 5 metre
tidal wave caused more havoc to the ruined commercial area. Texas City
was in ruins and the eventual death toll mounted to 576.
The chemistry behind this tragedy is very simple. The fertiliser was packaged
in paper bags. Paper is made from an organic plant material called cellulose.
The cellulose acted as a fuel while the ammonium nitrate acted as the
oxidant and eventual reaction was devastating. Click
to see how organic matter can act as a fuel in the presence of an oxidant.
The image on the right shows
the power of the blast as all the cars in nearby car parks were destroyed.

1. Ammonium nitrate is commonly used as
2. What are the common features of an explosion?
2. Why do so many explosives contain the element nitrogen?
4. One kilogram of petrol releases more than seven times the energy as
an equivalent mass of T.N.T. Yet T.N.T. is more explosive than petrol.
Explain.
5. How can petrol be made into an explosive mixture?
6. Describe the chemistry behind the Grandcamp incident.