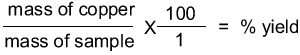Removal of copper from the solution. |
Filter the mixture as shown on the right and collect the filtrate in a beaker. The filtrate contains the dissolved copper as you can tell by its deep blue colour.
|
 |
| Thoroughly wash the insoluble substance left in the beaker so as to remove all the copper present. |
 |
| Place a long piece of zinc in the filtrate. Notice what happens. The copper will start to precipitate out of solution, as shown on the right. |
 |
Leave the zinc in the solution in a fume cupboard to react overnight. In the morning you should notice a clear solution, as shown on the right. This is because all the copper has come out of solution to form solid copper metal. |
 |
Using electronic scales, weigh a piece of filter paper. Filter the mixture. Make sure you wash the filter paper thoroughly using distilled water.
Why is washing the filter paper and copper important? |
 |
Scrape all the copper from the zinc metal onto the filter paper. Allow the filter paper to completely dry. You may wish to use a low temperature oven to help dry the filter paper.
Weigh the filter paper and the copper.
What is the mass of copper that was present in the ore sample?
|
 |
Calculate the percentage yield derived from the sample. |
|
| 1) Why are deposits of copper carbonate found near limestone deposits? |
| 2) Name five common copper ores? What is there chemical formula. Here is a start |
| 3) Outline the mining and recovery of copper on an industrial scale. |
| 4) Where is copper used in our society? |
| 5) Does copper mining damage the environment? Discuss with examples. |
| 6) Jonathon and Stephen were discussing ways of recovering copper from their solutions. Stephen mentioned that he could recover the copper by placing electrodes into the solution and passing an electric current through it. Jonathon then replied that placing a piece of zinc metal in the solution acts in much the same way. Explain |