The total energy of a system is made up of two types of energy, kinetic energy and potential energy. Kinetic energy comes from the movement of molecules in the gas and liquid phase or the vibration of particles in a solid. Potential energy is the energy stored in chemical bonds between atoms or molecules and comes about when bonds are brocken or formed.
When methane burns in oxygen, according to the reaction below, a great deal of energy is released.
![]()
This energy is released in the form of heat and is used to cook our food. The energy in the bonds of the products is less than the energy contained in the bonds of the reactants. It is safe to conclude that the products, in this case carbon dioxide and water, are more stable (have less energy) than oxygen and methane. The difference in energy between the reactants and products is given off as heat and light. This difference in energy is given the symbol below.
ΔH
This is known as the enthalpy
change and is measured by measuring temperature changes before and
after a reaction. A positive ΔH indicates an input of energy (endothermic reaction), while a
negative ΔH indicates energy
is given out(exothermic reaction).
Change in enthalpy is measured in kilojoules per mole.
Reactions that need energy to proceed are known as endothermic reactions. While those reactions that produce heat, such as the one on the right, are known as exothermic reactions.
.
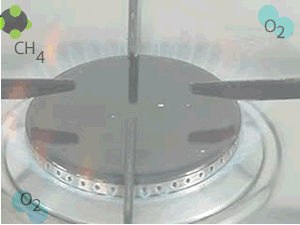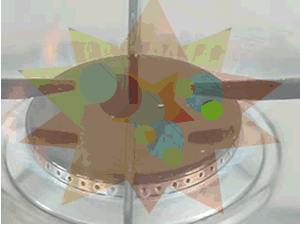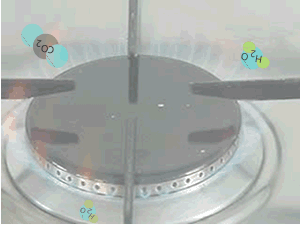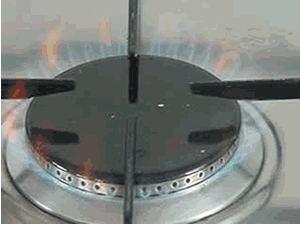
When methane reacts with oxygen,
a great deal of energy is released. The products are more stable than
the reactants.
Why is it that strong
collisions between hydrogen and oxygen molecules produce a reaction while
strong collisions between water molecules produce no reaction?
A simple class
room demonstration of the energy release of the above reaction can be
conducted. Click to see the procedure
The convention is that
exothermic reactions
are given a negative ΔH
while
endothermic reactions are given a positive ΔH.
Chemical equations written for exothermic or endothermic reactions show the enthalpy change.
CO(g) + 2H2(g) => CH3OH(g); ΔH = –90 kJ/ mol
Take the reaction of carbon monoxide with hydrogen gas, as shown above. For every mol of CO that reacts with 2 mol of H2 90 kJ of energy is given out (notice the negative ΔH value).
Click to continue with thermochemical equations.