Le Chatelier's Principle
If the temperature of the system
remains constant then the equilibrium position of the reaction will shift
to counter any changes made. Le Chatelier's Principle is a useful generalisation
that states " If an equilibrium system is subjected to a change
the system will adjust itself to partially oppose the effect of the change".
Lets see some examples.
The mixture of reactant and product molecules are at equilibrium. The straight line on the graph indicates the mixture is at equilibrium. Adding extra amount of reactant "A" forces the reaction forward in an attempt to partially remove the extra amount of reactant. As a consequence the amount of reactant "B" reduces while the amount of product increases. As a result of adding extra "A" the equilibrium expression below instantly decreases but returns to the original value once the system shifts in a net forward direction to partially absorb the extra "A" and reaches equilibrium once more. The value of K at equilibrium is known as the equilibrium constant (Kc)
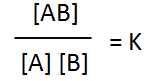
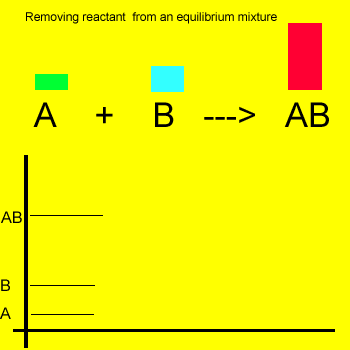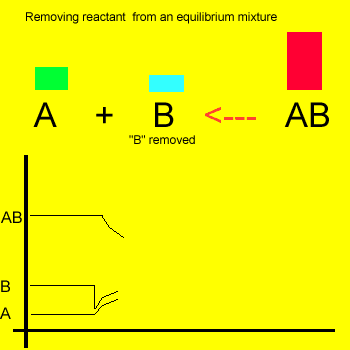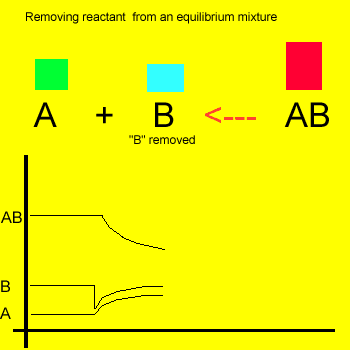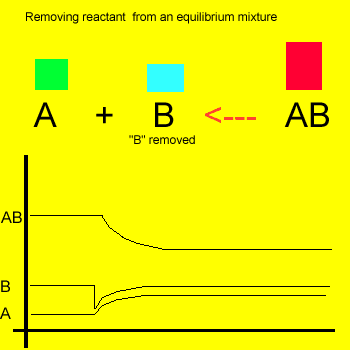
Removal of a substance from
the equilibrium mixture forces the reaction to shift so as to try and
form and partially restore the substance that was removed or decreased in amount. In the equilibrium
mixture on the left reactant "B" is reduced. The reaction therefore
moves to the left to compensate for the reduction and maintain a constant
value for the equilibrium mixture.
As a result the amount of reactant "A" increases while the amount
of product "AB" decreases.
The new equilibrium mixture will have the same equilibrium constant as previously.
ONLY temperature can change the equilibrium constant. At the same temperature therefore, the equilibrium constant will stay the same no matter what changes are made to the equilibrium mixture.