Equilibrium
activity.
The Fe3+ ion appears yellow in high concentrations but clear in low concentrations. SCN- ions are colourless while the FeSCN- ion ranges from brown (low concentrations) to deep red in high concentrations. Such a reaction gives us an opportunity to visualise how the equilibrium shifts when a reactant concentration is changed.
Look at the video of the removal of Fe(III) ions by the addition of NaF according to the reaction below.
Fe3+ (aq) + 6F- (aq) -> FeF63-(aq)
Explain how
the equilibrium shifts.
Why does the solution around where the crystals of NaF are added go
clear?
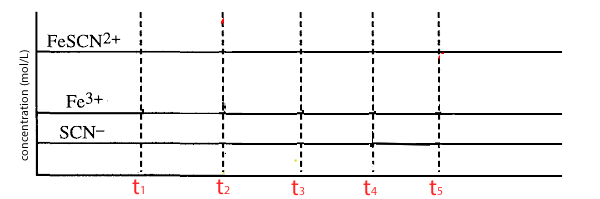
T1 = add Fe(III) ions. The system reaches equilibrium before T2
T2 = Add SCN- ions. The system reaches equilibrium before T3
T3 = Remove Fe(III) ions. The system reaches equilibrium before T4
Also include :
T4 = a small amount of distilled water is added. The system reaches equilibrium before T5
T5 = the equilibrium mixture is cooled.
Solution