When a metal atom is strongly heated, its electrons absorb the heat energy and jump to a higher energy level. When the electron returns to its original position it gives off the energy it absorbed in the form of light. Metal atoms give off distinct colours of light.
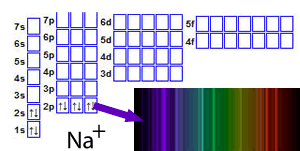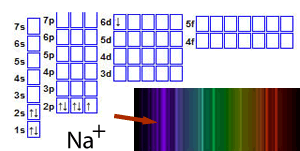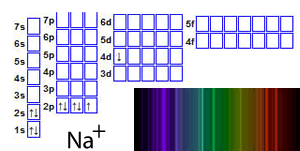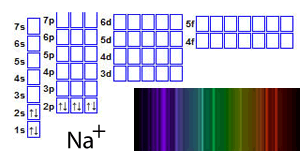
For example, a sodium ion in an unexcited state has the structure 1s22s22p6.
When the ion absorbs energy, electrons gain energy and jump into any of a number of empty orbitals at higher levels such as the 5s or 7p or 4d, depending on how much energy the electron has absorbed, as shown on the right
Now that the electrons are at a more energetically unstable state energy must be lost. Electrons tend to lose the energy they have absorbed by falling back to where they were before. This does not always happen in one go. For example, an electron that has been excited from the 2p subshell to the 6s subsell can fall back in one go or via another subshell, such as the 4d. This would release a certain amount of energy, relative to the jump, which would be seen as light of a particular colour. The result of all these jumps is to produce what is called an emission spectrum . An emission spectrum is a set of coloured lines that correspond to the energy the electron has released at each stage of its fall back to its original state. The combination of all these lines gives the metal ion a unique colour during the flame test.
Click to see the flame test of
lithiumpotassium
Sodium
Copper
-bunsen burner
-heat mat
-solutions of barium chloride,copper chloride, sodium chloride, strontium chloride and calcium chloride.
-spectroscope
-spray bottles

Spray each solution into the flame and record its colour. You may need to turn the light off in the classroom.

A method that is less messy and provides a long lasting coloured flame can be conducted usinga 2 litre soft drink bottle and a bunsen burner.
Click to see the setup.
View the video of this setup.

On the right is the flame for lithium chloride.
View the video of the lithium chloride flame test

On the right is the flame for copper chloride. Copper gives a green colour.
View the video of the copper chloride flame test

On the right is the flame for strontium chloride

On the right is the flame for sodium chloride
View the video of the sodium chloride flame test.
The colour of the flame may not be sufficient to distinguish between the different elements such as strontium and lithium. Look at the flame through a spectroscope to reveal the unique emission spectrum formed for each element. Click to see some examples of emission spectra.


