Relationship
between temperature, volume and pressure
for a fixed amount of gas
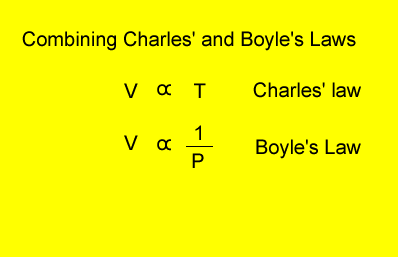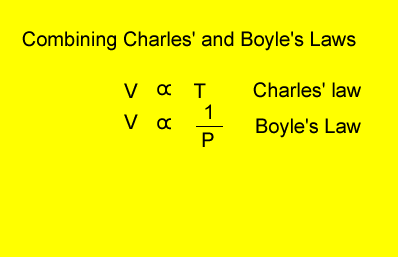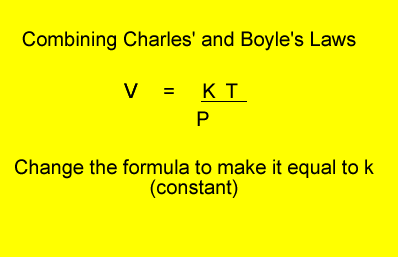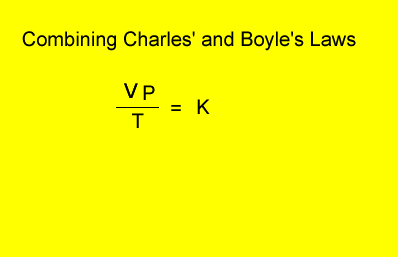
V = volume in litres
T = temperature in kelvin
P = pressure in kPa. Although units are not critical we will stick to using kPa.
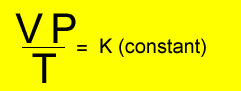
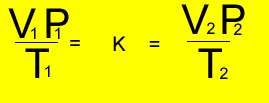
So let's put it to good use and see how it all works by trying some exercises below.
A fixed amount of gas was placed in a 6 litre vessel at 440 kPa at 30oC . The vessel was left in the heat and allowed to expand to 10.5 litres at a pressure of 490 kPa. What is the temperature of the gas?
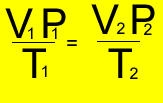

V1 = 6 L
T1 = (30 + 273 ) = 303K
P1 = 440 kPa
V2 = 10.5 L
P2 = 490 kPa
T2 = ?oK
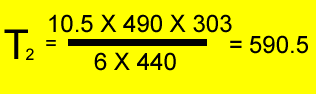
1)
A fixed amount of gas is placed in a 2.5 litre vessel at 20oC
and 95 kPa. The same gas is transferred into a 2 litre vessel at a temperature
of 40oC . What is the pressure exerted by the gas?
Solution
2) 400 ml of pure hydrogen gas was collected at 27oC and 90.5 kPa. It was later transferred into a 20 ml container at a temperature of -20oC. What is the pressure of the gas?
