Greenhouse Effect.
Ocean impact.
When the temperature is constant atmospheric CO2 is in equilibrium with dissolved CO2. As shown in the animation on the right, the amount of carbon dioxide in both the atmosphere and in the water stays the same because equal number of carbon dioxide molecules move into the water as move out of the water into the atmosphere.
The amount of gas that can dissolve in water is dependant on the temperature of the water. As the temperature of the water increases the amount of carbon dioxide that can be dissolved by a given volume of water decreases.
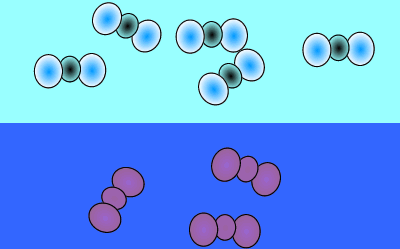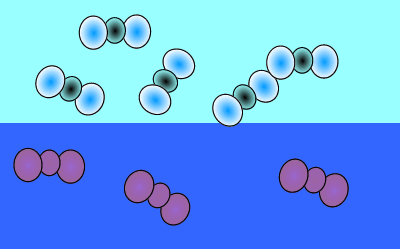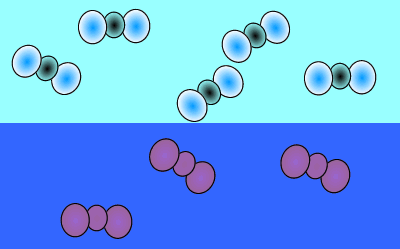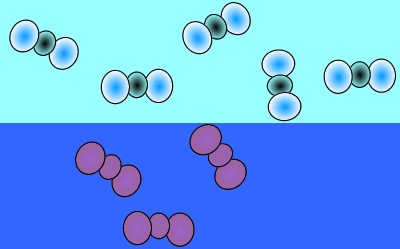
As atmospheric carbon dioxide increases it upsets the balance and causes an increase in dissolved carbon dioxide. This is achieved when more carbon dioxide molecules move into the water than move out of the water.
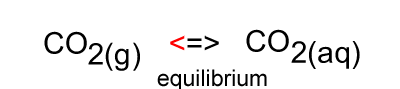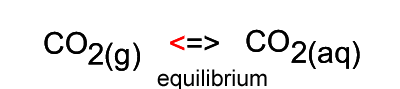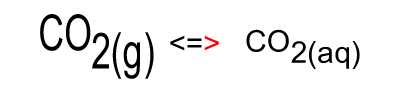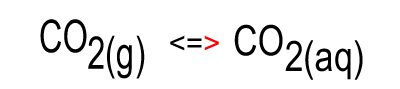
The image on the right shows the overall changes that occur when atmospheric carbon dioxide increases in concentration. Significant increases in amount of substance is indicated by an enlargement of the species.
Notice how an increase in dissolved CO2 causes an increase in carbonic acid (H2CO3 ). In turn this creates an increase in the concentration of carbonate (CO3-2 ) ions. Carbonate ions react with calcium (Ca+2) ions to produce limestone (CaCO3).
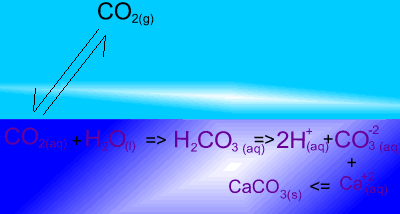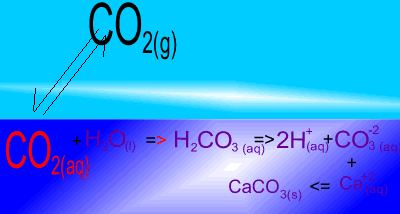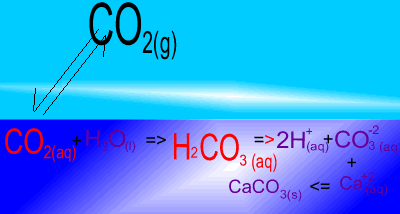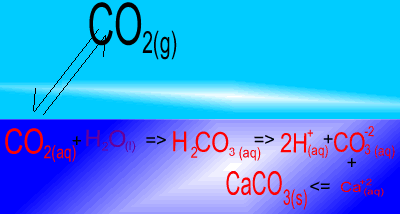
What would happen to the concentration of atmospheric carbon dioxide if the pH of the ocean decreases? Solution
What would happen to the pH of the ocean if the temperature of the water increases?
How will the amount of limestone deposited on the sea floor change if the temperature of the water decreased?