Exercises
|
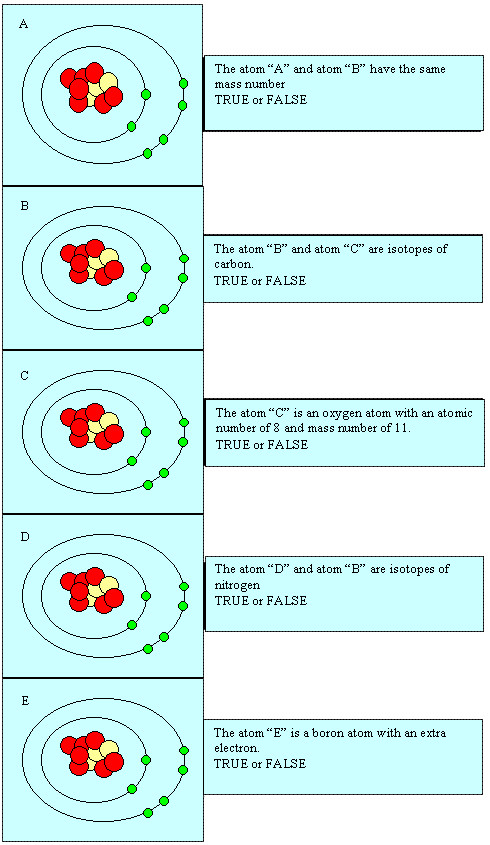
Atomic
nuclei
|
Comment
|
True
or False
|
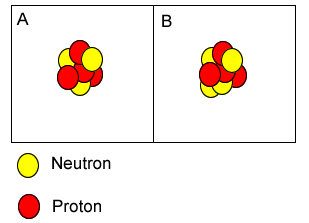 |
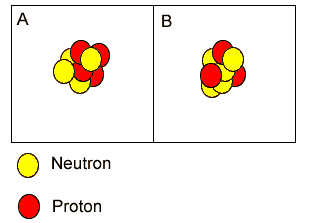
Atoms "A" and "B" are isotopes of berylium. | |
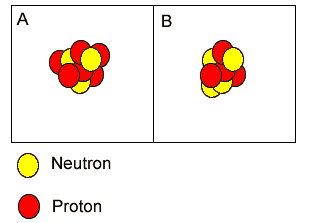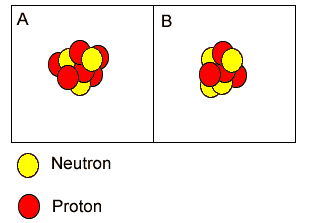 |
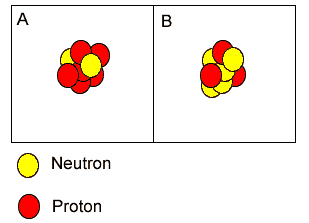
Atoms "A" and "B" are isotopes of nitrogen. | |
 |
Both atoms have the same atomic number but different mass number. | |
 |
"A" is carbon while "B" is a lithium atom. | |
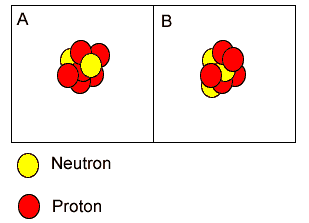 |
Both "A" and "B" are isotopes of boron because they have the same atomic mass but different atomic numbers. | |
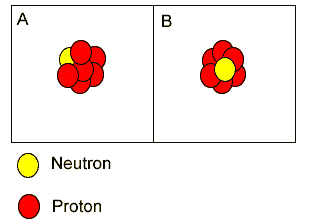 |
Both "A" and "B" are atoms of carbon. | |
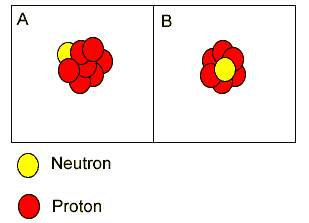 |
Both "A"
and "B" are isotopes of carbon.
|
|
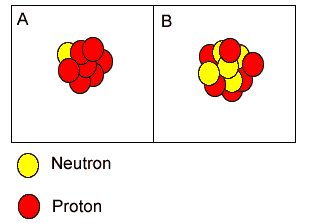 |
Both "A"
and "B" have the same mass number.
|
|
 |
Which nucleus is unlikely to be stable? The best reason for this is that: |