Hydrocarbons
Reactions
Alkanes,
in the gaseous state, will react with oxygen in highly exothermic reactions.
Methane burns in oxygen to release energy and produce carbon dioxide and
water. The reaction is shown on the right.
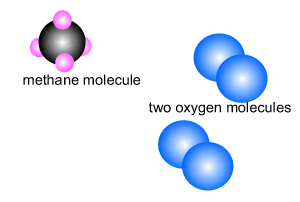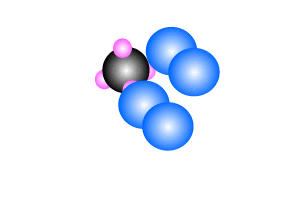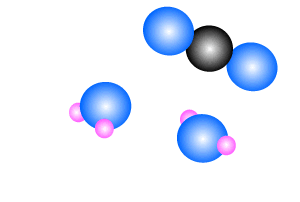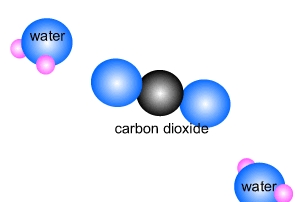
CH4(g) + 2O2(g) => CO2(g) + 2H2O(l)
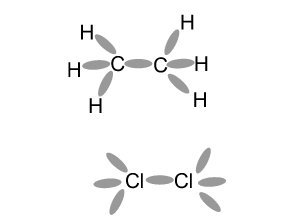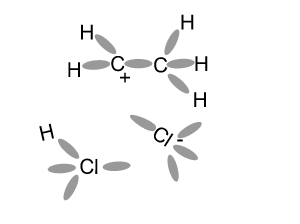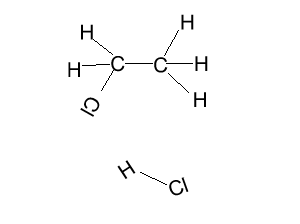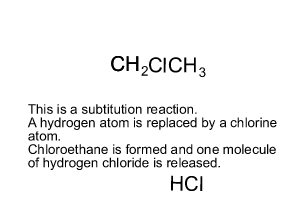
This may not be the exact mechanism for this reaction.
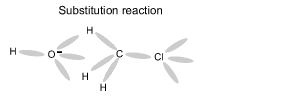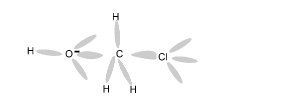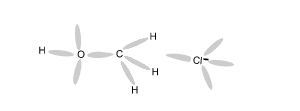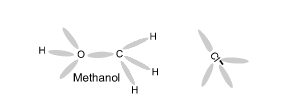
Using an electron dot diagram explain why a reaction between methane and chlorine gas can not produce the compound CH4Cl2 as specified in the chemical equation below.
CH4 + Cl2 => CH4Cl2
Is CH4 less soluble in water than CH3OH? Explain.
Compare the properties of ethane and ethanol.