Ester formation
Esters
are compounds formed from a reaction between an alcohol and an acid. Esters
have pleasant fruity smells and are used as artificial food additives.
Esters occur naturally in fruits. The natural esters give fruits their
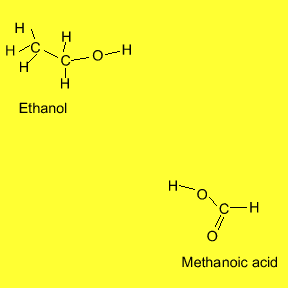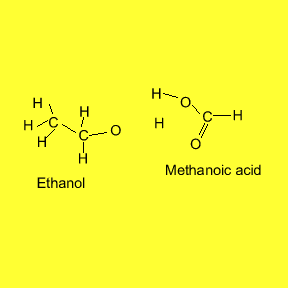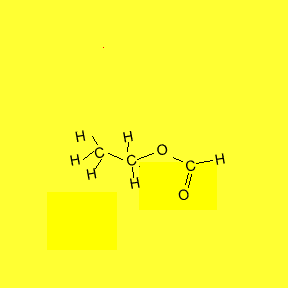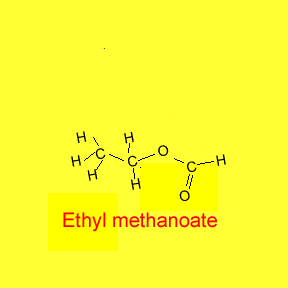
individual flavour and smell. The animation below shows the reaction between
ethanol and methanoic acid.
There is a certain methodology to naming esters.
Replace the
"anol" part of the alcohol with "yl"
in this case ethanol becomes ethyl
Replace the
"oic" part of the acid with "oate"
in this case methanoic
becomes methanoate
Placing the two names together we get
ethyl methanoate
Esters can also be formed into giant polymers. Polyester can be blow moulded into bottles and recycled to make fibres for use in carpets. Clothing made from polyester fibres are remarkably strong and resistant to creasing. The alcohol and acid monomers used in this condensation polymerisation reaction have two functional groups each and are able to to attach and grow at either end. Click to see an animation.
Experiment (ester formation)