Stereo-isomers- properties of cis and trans
Generally, cis and trans isomers have different physical properties that arise from differences in the shape of the molecule or the overall dipole moment. Lets first look at the boiling points of the cis and trans isomers. Cis isomers of a particular molecule have a higher boiling point than trans isomers of the same molecule.
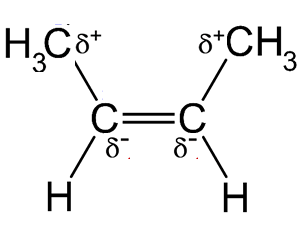
Now take the trans isomer. By comparison the van der Waals dispersion forces should be exactly the same, however, the trans molecule is non-polar as it is a symmetrical molecule.
The slight positive charge on the top right of the molecule, as shown on the right, has an equivalent positive charge at the bottom left of the molecule. The trans molecule is non -polar, unlike the cis isomer, the trans isomer does not have two poles, a δ+ and a δ- charge
This lack of overall polarity means that the only intermolecular attractions these molecules experience are van der Waals dispersion forces. Since less energy is needed to overcome these forces and separate the molecules, hence their boiling points are lower.
The size of the dipoles can be larger if more electronegative atoms are present, as in the case of 1,2-dichloroethene. The cis isomer in this case has a boiling point around 60 °C, while the trans isomer has a boiling point around 47 °C.
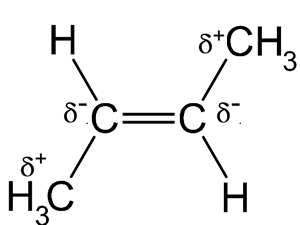
In the cis isomer the two delta negative chlorine atoms are found on the same side of the molecule and so combine to give an overall molecular dipole. This dipole is non-existent in the trans isomer and causes stronger intermolecular forces to exist , made up of stronger dipole-dipole bonding and dispersion forces similar to those found in the trans isomer. These stronger intermolecular forces, due largely to the dipole formation, account for the higher boiling point of the cis isomer.
When you look at the trans isomer such dipoles do not occur as the slight charge on the top of the molecule is exactly balanced by an equivalent charge at the bottom. Charges on the left and right of the molecule also balance, leaving a non-polar molecule.
Melting temperature is another thing, as a general trend, trans isomers have a higher melting temperature than their cis counterparts. The reason is not so much formation of dipoles but the ability of the molecules to pack in tightly in the solid state. Because dispersion forces and dipole-dipole bonding are relatively weak intermolecular forces they must act over very short distances. Molecules that can pack close to each other will experience a greater force of attraction than those that are further away.
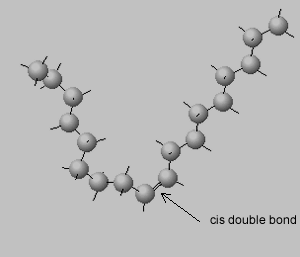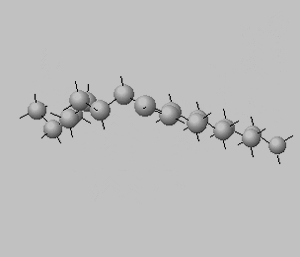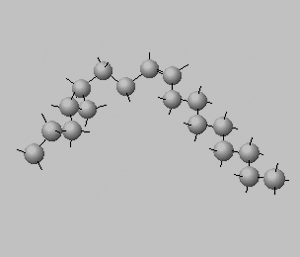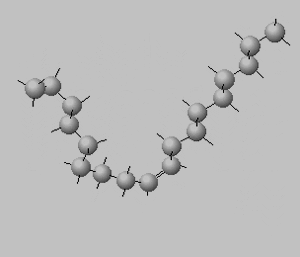
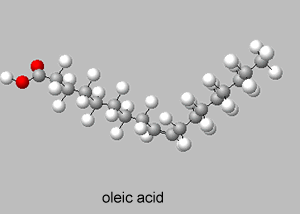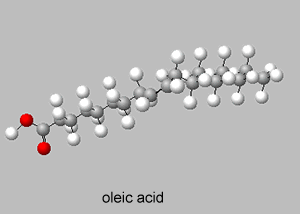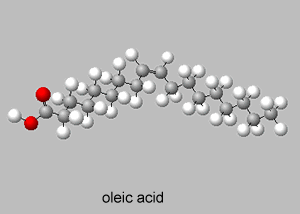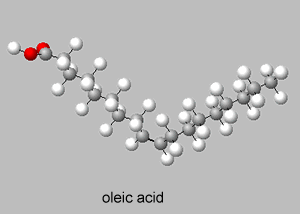
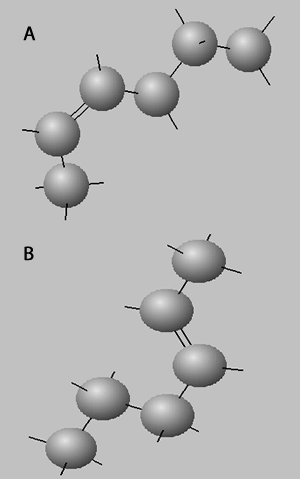
Trans isomers are more likely to be linear than the cis isomers. This is seen better when dealing with long carbon chains. A carbon chain, eighteen carbons long, is shown on the right with a cis double bond in the middle. Notice how the cis configuration causes the molecule to take up a "U" shape.
Trans isomers, on the other hand, are more linear. Shown on the right is the same eighteen carbon long hydrocarbon with a trans double bond.
Take the cis and trans isomers of but-2-ene for example. The cis isomer ,melts at -139 °C while the trans isomer melts at a higher temperature of -106 °C.
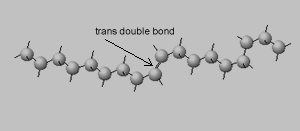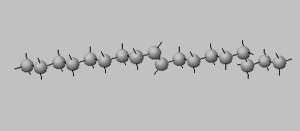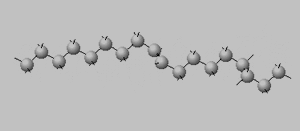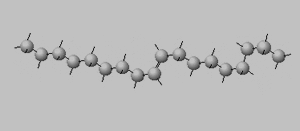
Oleic acid ( C18H34O2) is a liquid at room temperature with a melting temperature of 13.4 °C whereas elaidic acid (C18H34O2) is a solid with a melting temperature of 43.0 °C.
Oleic acid and elaidic acid are cis and trans isomers, the trans isomer packs in tightly in the solid state and hence has a higher melting point than the cis isomer.
Trans alkenes, therefore, are more symmetrical and hence less polar, have lower boiling points and higher melting points. Cis alkenes, on the other hand, are generally less symmetrical, and hence more polar, have higher boiling points and lower melting points.
1) Consider the molecules shown on the right.
a) Which molecules are polar?
Solution
b) Which are non-polar
Solution
c) Which molecule has the highest melting temperature out of trans-but-2-ene and ethene? Give an explanation
Solution
d) The molecule cis-but-2-ene, shown on the right, has a boiling temperature of 3.7 oC and metling temperature of -139 oC, while cis-2,3-dichlorobut-2-ene, also shown on the right, has a boiling temperature of 118 oC and a melting temperature of -27 oC. Explain the difference between the two molecules.
Solution
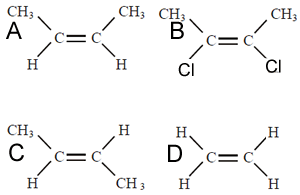
2) Consider the two structural formulas shown on the right. Which comments are true?
a) Molecule "A" has the highest melting temperature of the two.
b) Molecule "B" has the highest boiling temperature of the two.
c) "A"is less polar than "B".
Solution
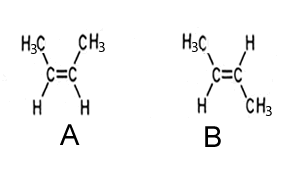
3) Consider the two stereo-isomers shown on the right.
i. Name each isomer
ii. One isomer has a boiling point of 67 oC and the other has a boiling point of 69 oC. Assign each isomer to the correct boiling point and give a reason.
Solution