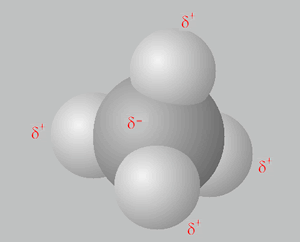
Solution
Why?
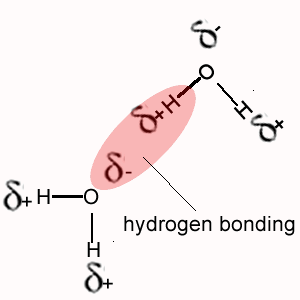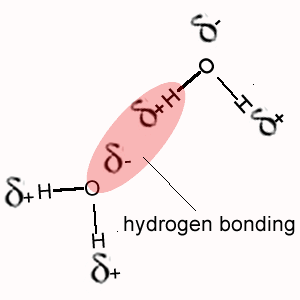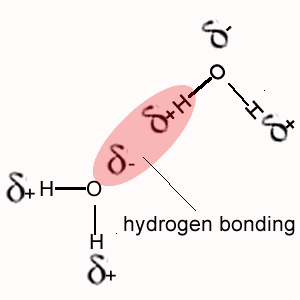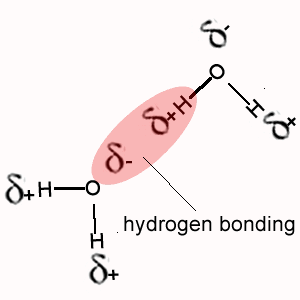
i. Methane (CH4) does not exhibit hydrogen bonding between its molecules as does NH4, H2O and HF.
ii. The Intermolecular forces are weaker in methane as it not only has no hydrogen bonding but its dispersion forces are also weaker due to the fact it is a smaller molecule than HF, NH4 and H2O.
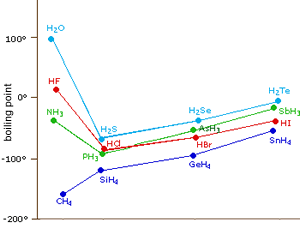

The polarity of the bonds formed when hydrogen bonds with oxygen, nitrogen or fluorine is much greater than the polarity of the bond formed between hydrogen and carbon.