1.An equilibrium mixture of four gases is represented by the following equation
![]()
The graph below shows the rate of the forward and reverse reactions versus time. A single change is made to the equilibrium mixture at time t1 and equilibrium is re-established at time t2 .
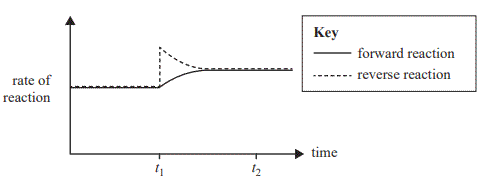
Which one of the following is consistent with the information given above?
A. Argon is added to the equilibrium mixture at time t1.
B. At time t1 reactants are removed from the equilibrium mixture.
C. The amount of products is higher at time t2 compared to just before time t1.
D. The change made at time t1 results in an increase in the equilibrium constant at time t2 .
2. Hydrogen, H2 , and iodine, I2, react to form hydrogen iodide, HI.
![]()
The graph below shows the concentrations of H2 , I2 and HI in a sealed container. One change was made to the equilibrium system at time t2 .
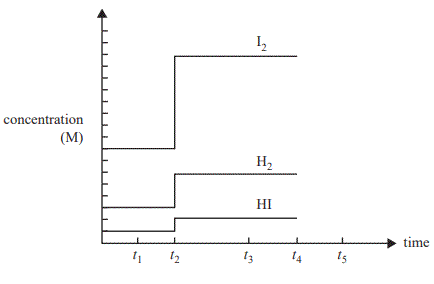
a. Which one of the following statements is correct?
A. A catalyst was added at time t2 .
B. The amount of HI is greater at time t3 compared with time t1.
C. The rate of reaction producing HI is the same at time t1 and time t3 .
D. The rate of production of HI at time t3 is double the rate of production of H2 at time t3.
b. One change was made to the equilibrium system at time t4 ,which altered the equilibrium constant. Equilibrium was re-established at time t5 . The rate of the reverse reaction at time t5 was higher than at time t3 . Which of the following options correctly shows the change in the equilibrium system from time t3 to time t5?
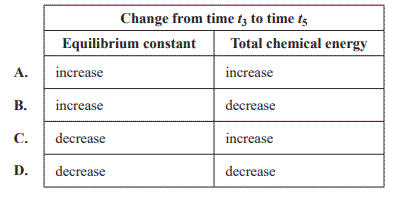
Solution

3. The reaction for the oxidation of sulfur dioxide, SO2 , is shown below.
![]()
a. 1.00 mol of SO2 and 1.00 mol of oxygen, O2 , are placed into an evacuated, sealed 3.00 L container at 100 °C. After the reaction reaches equilibrium, the container contains 20.0 g of sulfur trioxide, SO3 . Calculate the equilibrium constant, Kc, for this reaction at
100 °C
Solution

b. The graph below shows the Maxwell-Boltzmann distribution curve for the SO3 molecules in the container at a particular temperature. On the graph, draw the Maxwell-Boltzmann distribution curve of SO3 at a significantly lower temperature.
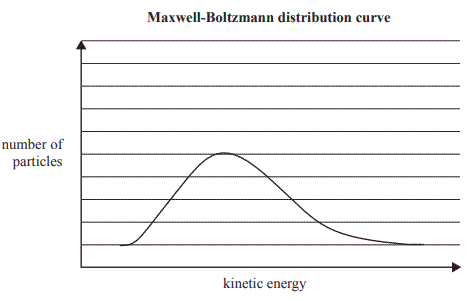
Solution

c. The volume of the closed container is doubled. Describe the effect that this has on the concentration of SO2 from the time just before the volume was changed until after the system re-established its equilibrium. 3 marks
.
Solution
