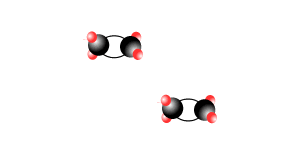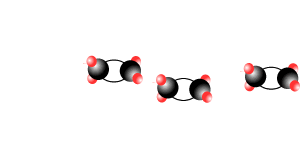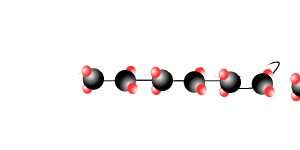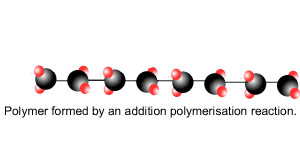Polymerisation
Addition and Condensation
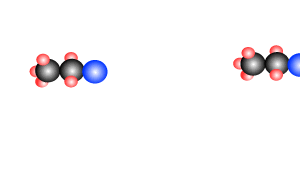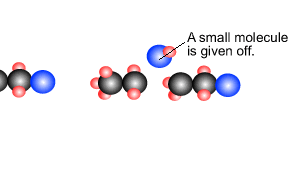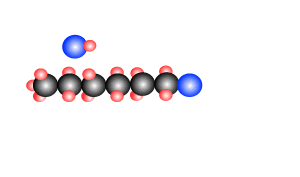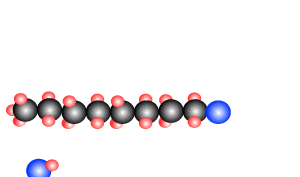
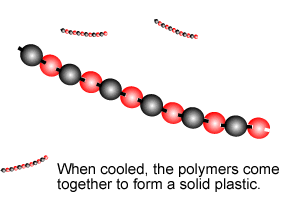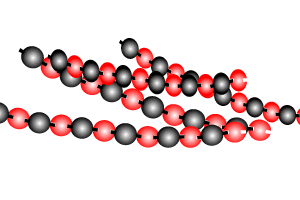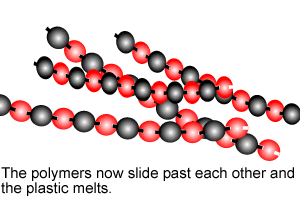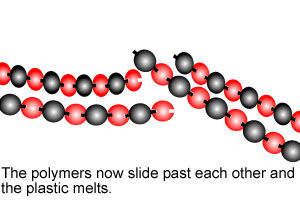
Polymers are giant molecules formed when smaller molecules, known as monomers, link together during the chemical reaction known as polymerisation.
There are two types of polymerisation
reactions, addition and condensation. These are discussed briefly below
in a simple manner for junior science students.
For a more detailed description of polymerisation visit the Organic
Chemistry section.
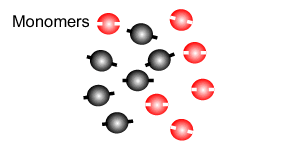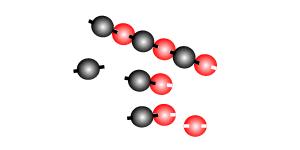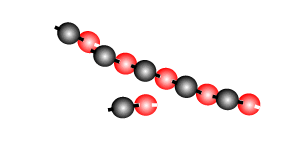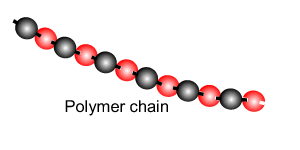
Addition polymersiation involves the linking of monomers with double bonds. The double bond of one monomer breaks and links onto the neighbouring monomer. This process continues, as pictured on the right, to form polymers, some of which can be many thousands of monomers long.
The animation on the right is of ethene molecules linking together to form polyethene.