The animation below reveals most of the answers to the problem.
It is easier to
see which species is the oxidant and which is the reductant if we place them
in order of oxidant strength according to the half cell potential.
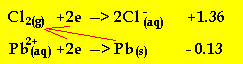
The arrow indicates that reductant (Pb) will react with an oxidant (chlorine
gas) above it on the electrochemical series.
The cell potential difference = 1.36 - -0.13 = 1.49V
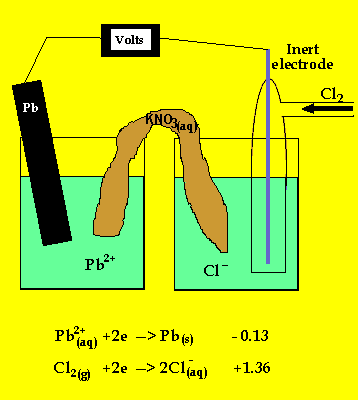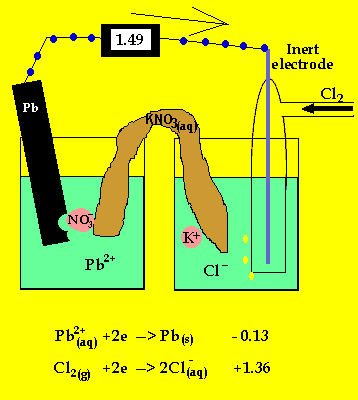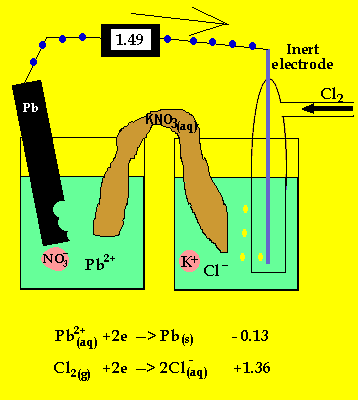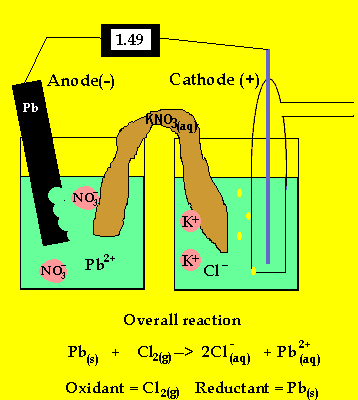
The anode is where oxidation takes
place and in a galvanic cell this has a polarity of (-). In this case the
anode is formed by the lead electrode.
The inert electrode forms the cathode(+) as this is where reduction (electron
usage) occurs.
Hide solution
