Galvanic cells.
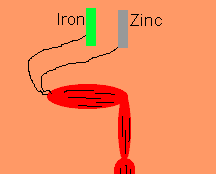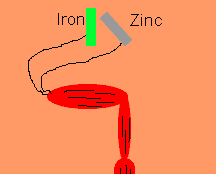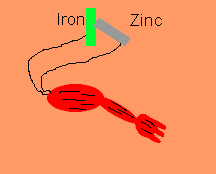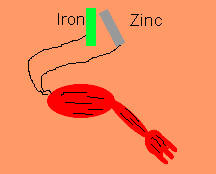
Luigi Galvani discovered, in 1791, that frogs legs can be made to twitch by connecting the nerve and muscle tissue to different metals such as zinc and iron. He thought that he had discovered a new form of electrical energy. He called this new energy "animal electricity". What he had discovered was the chemical link between electricity and chemical reactions. Galvani's name lives on in terms such as galvanised steel and galvanic cells.
A galvanic cell consists of two half cells. One half cell contains the oxidation (electron producing) reaction while the other contains the reduction(electron consuming) reaction. The two half cells are connected by an external circuit. Electrons are forced to flow through this external circuit passing through items such as a light globe and doing useful work.
Shown on the right represents a special type of galvanic cell known as the Daniell cell. This was the first cell to find large scale commercial use. Modern batteries work in much the same way.
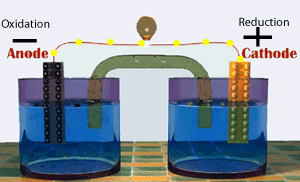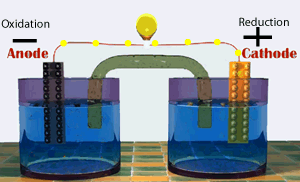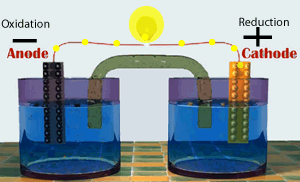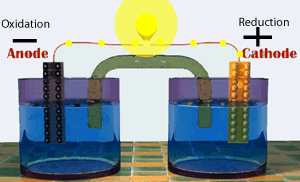
Continue with a discussion on the production of electrical energy from chemical reactions.