Hall Heroult cell
Aluminium metal is extracted from aluminium oxide, commonly known as alumina (Al2O3)
Alumina is extracted from bauxite which is dug out from the ground. Bauxite is an ore largely composed of gibbsite Al(OH)3, mixed with iron oxides and clay minerals.

Alumina melts at temperatures of 2,050 oC. Such temperatures are very expensive to maintain from a commercial point of view.
However, alumin readily dissolves, when placed in molten cryolite (Na3AlF6) at significantly lower temperatures of about 950 oC.
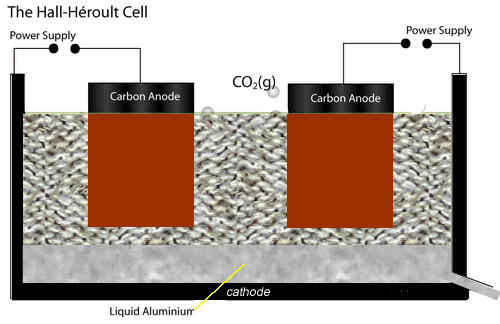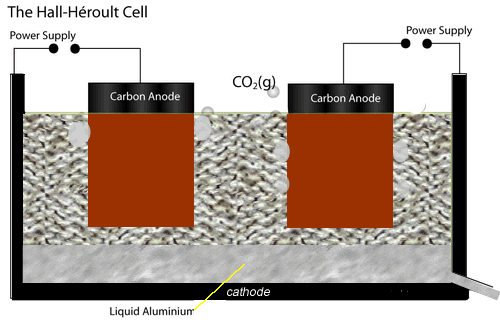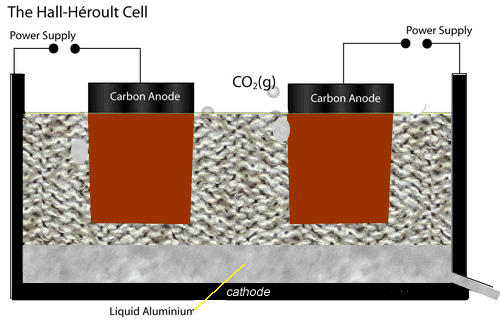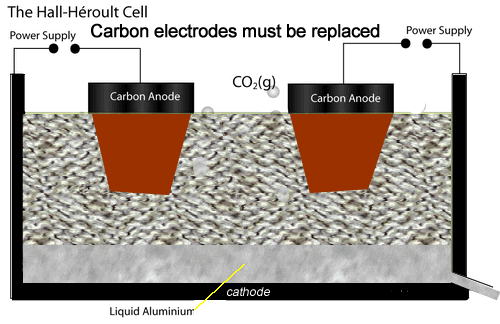
The electrolytic cell used to extract aluminium metal is represented by the diagram shown on the left.
Both the anodes and cathode are made of carbon conducting a current through the electrolyte, cryolite and alumina mixture, of about 150,000 A at 5 V.
The current passing through the electrolyte produces enough heat to keep it molten.
At the cathode aluminium ions are converted to aluminium metal. Click the blue writing for more detail.
While at the anodes, oxide ions are reduced to oxygen gas which quickly reacts with the carbon electrodes to produce carbon dioxide gas. Hence the anodes have to be replaced at regular intervals.

The overall reaction is given below.
2Al2O3(in cryolite) + 3C(s) => 4Al(l) + 3CO2(g)
1) Cheap electricity is essential for the commercial viability of an aluminium smelter.
a) Calculate the electric charge required to produce 0.500 kg of alluminium from alumina in Hall-Heroult cell.