.A reductant is an electron donor.
An oxidation reaction involves a reductant and the production of electrons hence electrons appear as products. Common in all oxidation reactions is an increase in charge number, commonly known as oxidation number, of one of the species.
An oxidation reaction is written as a balanced half-equation
Take the following oxidants as examples.
- Calcium metal is a powerful reductant.
- Lithium metal is also one of the strongest reductants and readily gives up electrons therefore it is extensively used in batteries.

An oxidant is an electron acceptor.
A reduction reaction involves an oxidant and the use of electrons hence electrons appear as reactants. Common in all reduction reactions is a decrease in charge number, commonly known as oxidation number, of one of the species.
A reduction reaction is also written as a balanced half-equation.
Take the following oxidants as examples.
- Chlorine gas is a powerful oxidant.
- Iron (Fe2+)
ion also acts as an oxidant
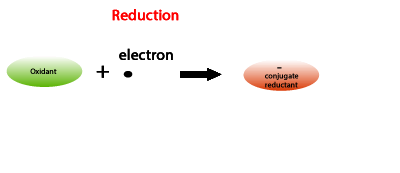

The reductant forms its conjugate oxidant while the oxidant form its conjugate reductant.
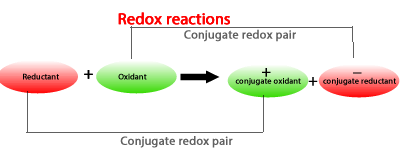
Lets see an example.
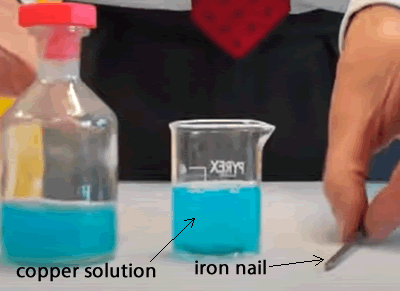
When an iron nail is placed in a solution of Copper(II) ions a pink coating of copper quickly forms on the iron. This is an example of a redox reaction.
Click to see the
equation to the overall reaction.
The equation to the overall reaction is formed by adding the oxidation and the reduction reaction equations.
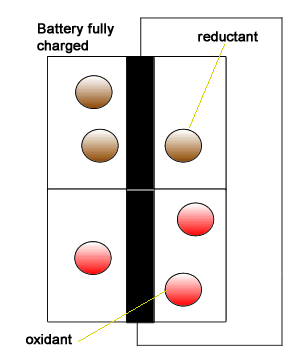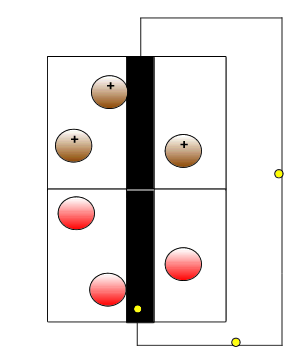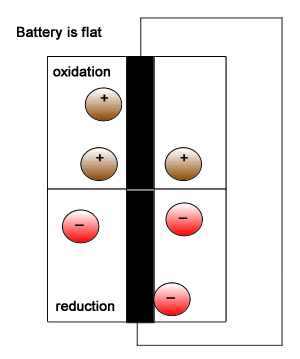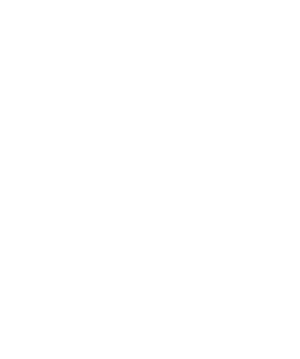
Redox reactions are part of batteries and fuel cells that drive our electric vehicles. Scch reaction provide electrical energy to power heavy machines and our communication devices.
The animation on the right gives a simplistic view but highlights the need to have the reductant and oxidant isolated and connected only by an external circuit through which electrons flow.