Precipitation with solubility curves
When a saturated solution is cooled crystallisation of the solute occurs. A saturated solution of compound "X" was formed at 50 0C by the slow addition of "X" to 100 grams of water. The solution was then cooled to 40 0C. What mass of X is precipitated?
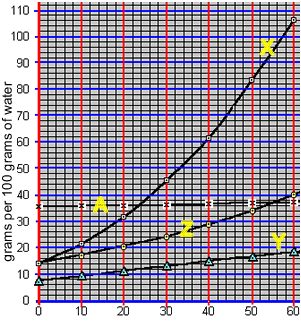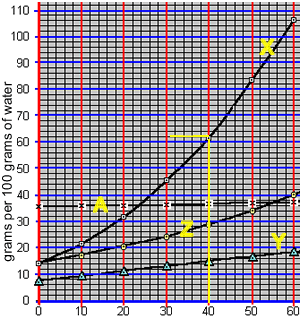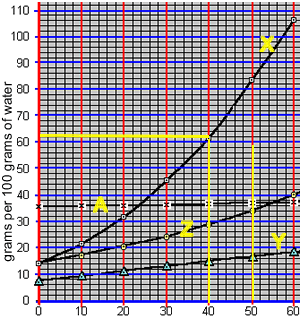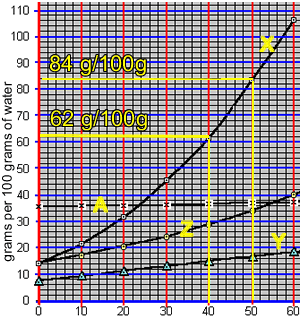
According to the animation on the right, 50 0C 100 grams of water can hold 84 grams of compound "X". However, at 40 0C 100 grams of water can only hold 62 grams of the compound.
The difference will precipitate out of solution. So, 22 grams of "X" will precipitate out of solution.
1) 100 grams of water at 30 0C was saturated with compound "Z". The solution was then cooled to 200C. What mass of compound "Z" precipitated out of solution?
2) 50 grams of water at 50 0C was saturated with compound "Z". The solution was then cooled to 300C. What mass of compound "Z" precipitated out of solution?
3) 100 grams of water, at 60 0C, was saturated with compound "X". The solution was then cooled to 40 0C. What mass of solute precipitated out of solution?