Precipitation reactions
When two solutions are mixed, sometimes, an solid is formed that comes out of the solution. This solid is generally known as a precipitate. Click to see lead iodide precipitate being formed. Precipitates have very low solubility in water.
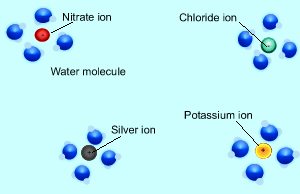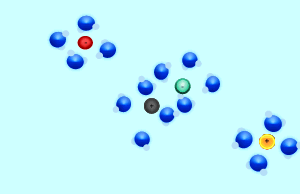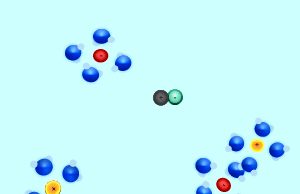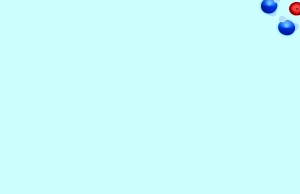
Consider the formation of silver iodide from the mixing of the solutions silver nitrate and potassium iodide. The reaction is shown on the right. When the two solutions are mixed some of the ions in each solution react together. In this case the silver and the iodide ions react to form solid silver iodide. Ions that do not react are known as spectator ions.
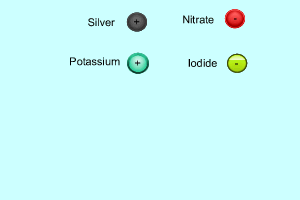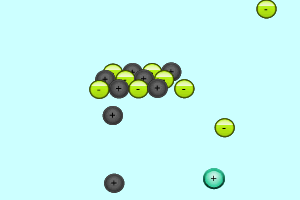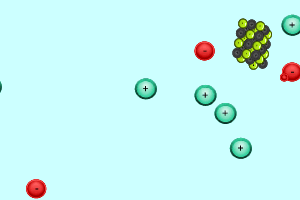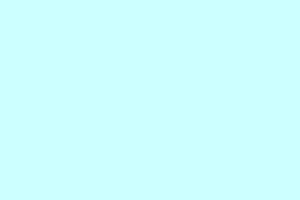
When charged particles (ions) are placed in water, water molecules are attracted to them by electrostatic forces and surround each ion. In order for two ions to combine together and form a precipitate they must overcome the forces attracting them to the water molecules.
Consider the animation on the right. The silver and chloride ions have a strong attraction and are able to overcome the forces that bind them to the water molecules. They react together to form a solid which separates from the solution. Notice how the potassium and nitrate ions can not overcome the forces of attraction with the water molecules.