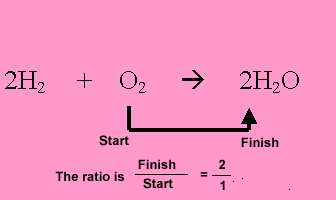
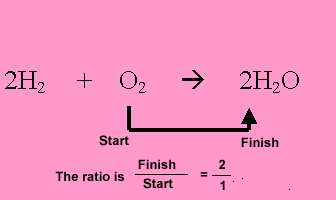
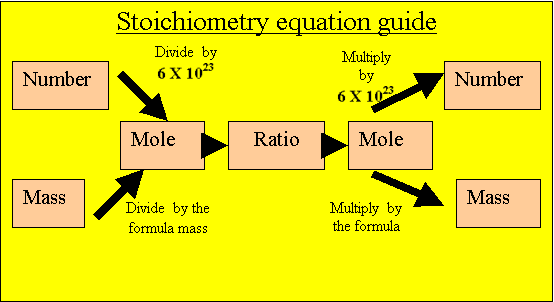
Convert the mass of oxygen gas into moles of oxygen gas.
16 grams/ 32 atomic mass units.
= 0.5mole of oxygen molecules
0.5mole of hydrogen molecules are present to react.
Caclulate the mole of water that will be produced from the
hydrogen present. Multiply the mole of hydrogen by the ratio.
2/1X 0.5= 1mole of water.
Calculate the mass of water
Multiply the moles of water by its formula mass.
1 X 18 = 18grams.
|
Limiting and excess reactants exercise
|
|
Hydrogen reacts with oxygen to form water according to the equation below. 2H2 + O2 => 2H2O
We now use the oxygen to work out the mass of water produced.. |
|
Double Click anywhere in the green region of the document to see the working out. Click on the squares inside the diagram to reveal more information. Double click to remove the information. |
|
|