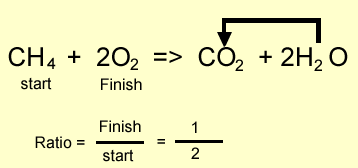
Divide the mass of water (3.6 grams) by its formula mass.
Formula mass of water is 18.
The number of moles of water is therefore given by the expression
3.6/18 = 0.2 mole
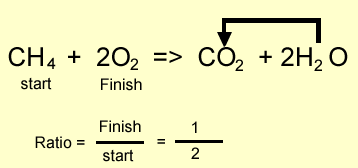
The number of mole of carbon dioxide are calculated as shown below.
mole of water X ratio = mole of carbon dioxide
0.2 X 0.5 = 0.1 mole of water
Calculate the mass of carbon dioxide. The mass of carbon dioxide is given by the expression below.
mole X formula mass = mass
The formula mass of carbon dioxide is 44, therefore the mass of carbon dioxide is 0.1 X 44 = 4.4 grams
|
Mass-mass stoichiometry
|
|
Methane burns in oxygen to produce carbon
dioxide and water according to the equation above.
Step 2 Calculate the ratio from the prefixes in the balanced equation above
Step 3 Calculate the number of mole of carbon dioxide formed.
Step 4 Calculate the mass of carbon dioxide.
|
|
|