Volumetric analysis
exercise 7
|
Volume
|
|
| Start |
15.40
mls
|
| Finish |
32.22
mls
|
| Total |
16.82
mls
|
A 0.15gram sample of hydrated sodium carbonate was dissolved in 20 mls of water. The solution was then titrated against a 0.2M HCl solution. The result is shown on the left.
What mass of the sample is due to water?
( Atomic mass of Na = 23, H = 1, O =16, C =12)
The equation for the reaction is shown below.
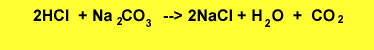
Step 1 Calculate
the mole of substance delivered from the burette. In this case HCl solution
Mole = Average Volume(litres)
X Concentration
Mole of HCl = 0.0168 X 0.2 = 0.00336 mole
Step 2 Using
the equation calculate the number of mole of sodium carbonate in the
sample.
Mole of sodium carbonate = 0.00168 mole
Step 3 Find
the mass of sodium carbonate present in the sample.
Mass of sodium carbonate = 0.00168 X 83 = 0.139 grams
Step 4 Calculate the mass of water in the sample.
Mass of water = 0.150 - 0.139 = 0.011 grams