Volumetric analysis
exercise 9
4.00g of an unknown triprotic acid was placed in a volumetric flask and made up to 250ml of solution. A 20ml aliquot was taken and titrated against a 0.109M NaOH solution. The result is shown on the right
Calculate the relative molecular mass of the acid
|
Volume
|
|
| Start |
15.40
mls
|
| Finish |
32.22
mls
|
| Total |
16.82
mls
|
The equation for the reaction is shown below.
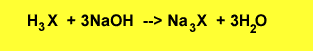
Step 1 Calculate
the mole of sodium hydroxide
Mole = volume X concentration
Mole = 0.0168 X 0.109 = 0.00183 mole of NaOH
Step 2 Using
the equation calculate the number of mole of acid required to react
completely
For every mole of NaOH used a third of a mole of acid reacts.
0.00183 / 3 =0.0006 mole of acid in the 20ml aliquot
Step 3 Find
the molecular mass of acid
Molecular mass = mass
/ mole
FIrstly find the mass of acid in the 20ml aliquot.
4.00 grams X 20/250 = 0.32grams
Molecular mass = 0.32 / 0.0006 = 533.3 mass units.