The water molecule is made up of three atoms. Two hydrogen and one oxygen. This arrangement of atoms makes the molecule very polar. That is, it has small charges on both ends. These small charges attract each other relatively strongly and hold the molecules very close together.
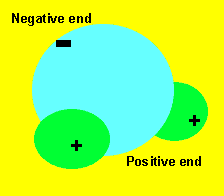
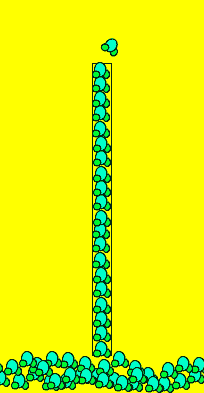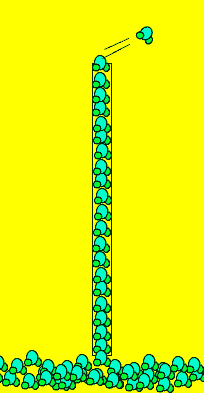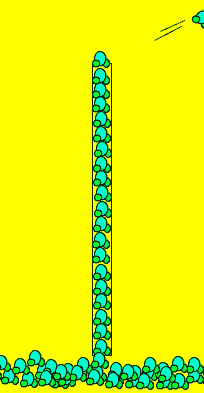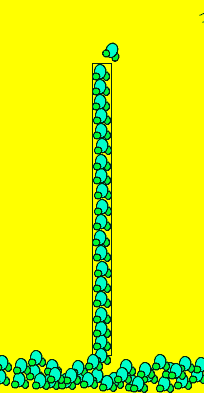
The small charges on the water molecule give it special and unique properties. Water is the only substance that expands when cooled and can move up through tiny tubes for hundreds of meters. The abiloity of water to rise through tiny gaps is known as capillary action. Often you will see water rising up walls when there is a drainage problem.
Capillary action allows trees to get water to their leaves and is the reason why water soaks quickly through paper and clothing. Due to the charge on the molecules, they line up end to end with the negative end of one molecule facing the positive end of the one in front of it. This attraction is strong enough to lift long thin collumns of water many metres high. If the column of water becomes too wide the mass of the column can not be supported by the force of attraction between the charges. This is why you never see water slowly rising up the inside of a glass, it is too wide. Not only do the water molecules attract each other but they also attract and hold onto the sides of the tiny gaps they move through. This also helps support the column of water molecules.
Click to see an activity demonstrating the presence of charges on the water molecules. Notice how the column of water bends when a charged object is brought close.
