Nuclear Power
Glossary of terms you may have to be familiar with.
Atoms with large nuclei, such as uranium, tend to be unstable. Occasionally an atom will split into two smaller atoms and in the process give off a great deal of energy in the form of heat and light.
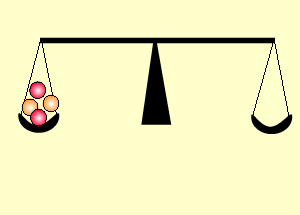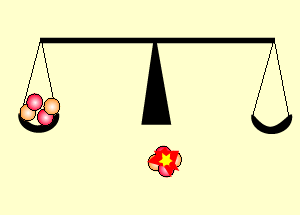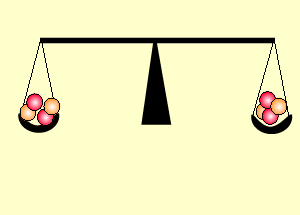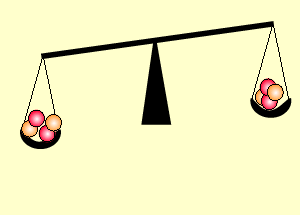
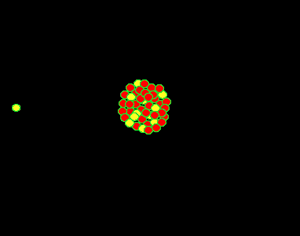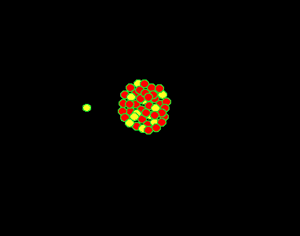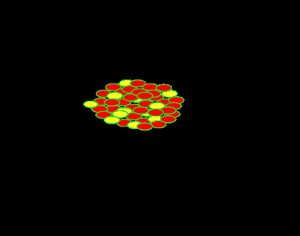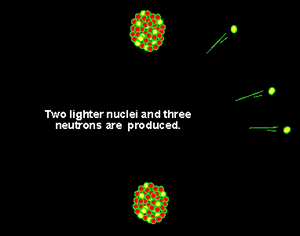
Uranium is the fuel used
to power nuclear reactors. It is a heavy metal which is mined in many
countries including Australia. 99% of uranium is composed of the U238
isotope while the U235 isotope makes up 0.7% of the natural
uranium found on earth. U235 is used for the production of
nuclear weapons and as a fuel for nuclear power production. This particular
isotope is one of the few substances that will undergo nuclear fission
when bombarded by neutrons. When struck by a neutron, the U235
nucleus absorbs the neutron, becomes unstable and splits into two smaller
nuclei with the evolution of a great deal of energy according to the equation
E = mc2.
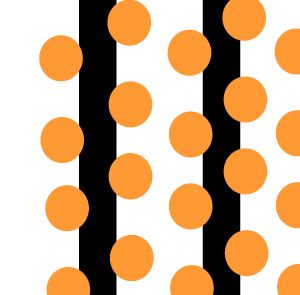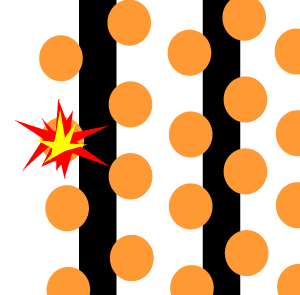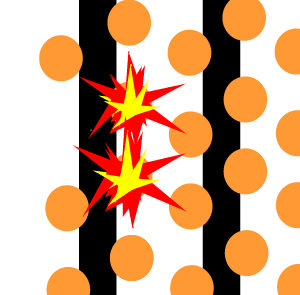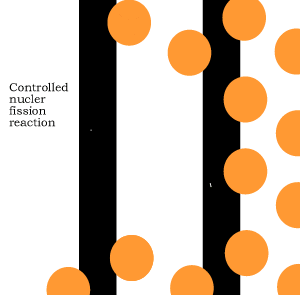
The animation on the right shows an enriched piece of uranium, with up
to 3% U235, undergoing fission in a nuclear reactor. The control
rods (shown in black) absorb neutrons.
For the production of nuclear
weapons 90% enrichment is required. That is 90% of the uranium atoms in
the fuel must be U235. Construction of a nuclear bomb is not
difficult, the difficulty lies in the separation of U235 from
the rest of the uranium. It took the Manhattan Project scientists 2 years
to gather enought U235 to use in the bomb detonated over Hiroshima.
The 3% enriched uranium
is packed into small circular pellets about 2 cm in diameter and 2 cm
long. These are arranged into long rods that are collected together in
bundles. These bundles are lowered into the reaction vessel and immersed
in water, which acts as a coolant. If left on its own the fission reaction
would proceed and eventually overheat and melt the reaction vessel.
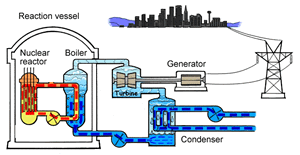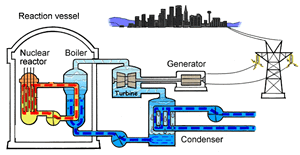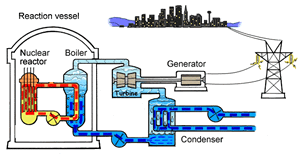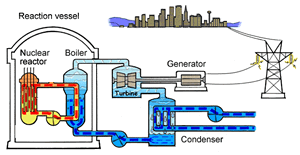
To prevent this melt down from occurring, control rods are inserted into the reaction chamber. These rods are made of a material that absorbs neutrons and so can slow the rate of the fission reaction. For example, if an operator wishes to increase the temperature of the reactor they will raise the control rods out of the reaction chamber. If they wish to decrease the temperature they will lower the control rods further into the reaction chamber until the rate of the reaction ceases.
Look at the animation above. The more control rods present in the reaction
chamber the slower the fission reaction and hence the slower the rate
of energy production.
A nuclear power station looks very similar to a normal coal fired power station. You can see on the right the huge chimneys, condensers, used to cool the superheated steam back to water.
picture from Google.

The major difference inside a nuclear power station is the way heat is generated in order to create super hot steam. Heat is created in the nuclear reactor through nuclear fission. The heat is then used to turn water into steam which is used to drive the generators to produce electricity.
picture from Google.
Why is it unlikely that a nuclear reactor can explode like a nuclear bomb?
What is the difference, in chemical behaviour, between the U235 isotope and the U238 isotope?
Why is the U235 isotope used to make a nuclear bomb and not the U238 isotope?
What is the role of control rods in a nuclear reactor?