Rocket fuels
A chemical decision
5 grams of each fuel was tested by burning in pure
oxygen

Fuel “A” is a solid which
spontaneously combusts in the presence of oxygen. In the absence of pure
oxygen fuel “A” is extremely stable.
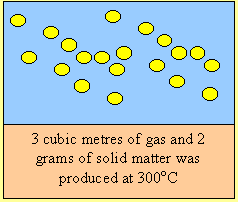

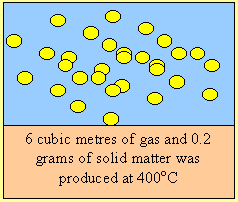
Fuel “B” does not react with oxygen in the air, however in the presence of a catalyst, it reacts violently producing carbon dioxide and water vapour. It’s boiling point is –10C, it is very corrosive and must be stored as a liquid

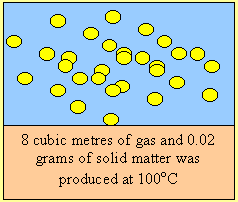
Fuel “C” is spontaneously combustible when mixed with oxygen.
It reacts readily with air to form an explosive mixture of gases. It is
safe when stored at -100C.
1) A fellow scientist turns to you and says “The fuels used in rockets must undergo EXOTHERMIC reactions when combined with oxidisers”. Do you agree? Research what is an exothermic reaction and an oxidiser and offer an explanation
2) What is a catalyst? Does it have any special properties?