Air
is made up of different tiny molecules. These molecules constantly collide
with surfaces. The surface of your skin sustains billions of collisions
every second. The molecules are so small that we hardly feel the impacts.
Take a glass window for example, the number of collisions on the outside
of the window equal the number of collisions on the inside. This balance
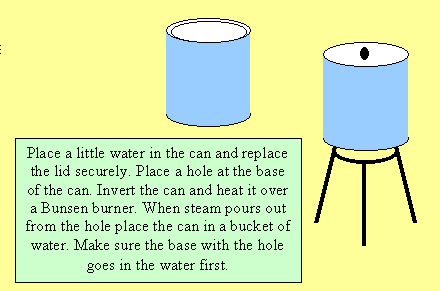
prevents the glass from being smashed by the air pressure. Conduct the
following demonstration where the number of collisions on the outside
of the can far exceed those on the inside.

Click to see a 120Kb video of the imploding
can.