Little packets of energy
“Energy cannot be created nor can it be destroyed. It can only change from one form into another”
Before we discuss the energy stored in atoms and molecules let's recap the structural nature of the atom.

A more violent release of chemical energy takes place when hydrogen and oxygen are ignited. During this chemical reaction, hydrogen and oxygen molecules react with each other to form water and in the process release a great deal of energy that characterizes the explosion shown in the video.
The energy is held in the chemical bonds that hold atoms together in the molecule. DUring a chemical reaction molecules break apart and their atoms recombine to form new substances. These substances have atoms held together by chemical bonds with less energy than the reactants. The excess energy is given off as heat, light or sound.
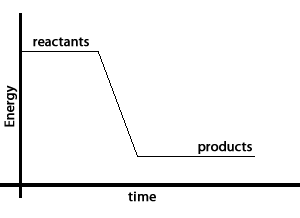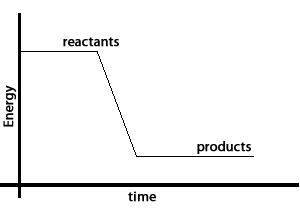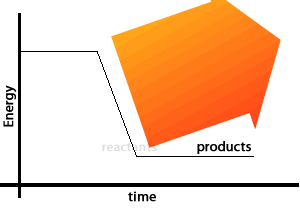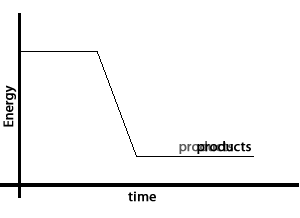
Take the reaction between hydrogen gas and oxygen gas to form water. The water molecule contains less energy than the hydrogen and oxygen molecules that created it. The excess energy is given off as heat and light. This is shown by the energy graph on the right.
Chemical bonds that hold the hydrogen and oxygen atoms together in the water molecule have less energy than the bonds holding the molecules that formed water together.
Molecules with a great deal of energy in their electrons usually prefer to react with other molecules to try and reduce that energy. Molecules, such as nitroglycerine, have a great deal of energy in their bonds and are said to be unstable. Such molecules may react spontaneously or with other molecules to release a great deal of energy.
Such reactions can release so much energy that they are used to launch huge spacecraft into space or as explosive devices.

So where does the energy to lift the shuttle come from?
A good fuel should contain chemical bonds with
It should then react to form substances with as little energy as possible. Explain why
Good fuels produce gaseous substances when they react. Why is this essential in creating thrust?
