The dating of older, nonliving, things is accomplished with radioactive minerals such as uranium. Isotopes of uranium decay very slowly to become isotopes of lead. For example, the naturally occuring isotope uranium-238 decays to become lead-206. Uranium-235 decays to lead-207 and not to the common isotope lead-208. So all the isotopes of lead-207 and lead-206 present in a rock sample were at one time uranium. The older the uranium-bearing rock the higher the percentage of these lead isotopes.
Knowing the half-lives of the uranium isotopes and the percentage of lead isotopes in the uranium-bearing rock we can calculate the age of the rock.
Uranium-235 has a half-life of 710 million years where as uranium-238 has a half-life of 4.5 billion years. The useful range for this type of dating is between 10 million and 4.6 billion years.
The percentage of lead and uranium isotopes can be determined by mass spectroscopy. This technique reveals the mass of each isotope present and its percentage composition. The mass spectrum on the right reveals the percentage abundance of uranium-238 and lead-206.
We can now calculate the age of the rock. A 12.5% decay of uranium-238 to lead-206 represents a quarter of a half-life. This equates to 1.1 billion years. Any fossil found in this rock can be dated to approximately 1.1 billion years old.

Calculate the age of the rock whose mass spectrum is shown on the right.
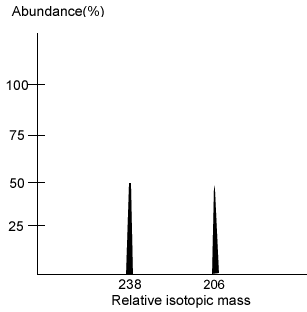
What isotopes accumulate in uranium bearing rock?
A uranium bearing rock was analysed and was found to contain the isotopes uranium-238 and lead-206 in the ratio 4:1 respectively. How old is the rock?