Lemon Battery
and the reactivity of metals
For this activity you will need:
-
a multimeter or some way to measure current or voltage;
- Wires with alligator clips attached
- 20 lemons or lemon juice
- 4 X beaker (50 mL)
- metal strips of copper, zinc, lead, nickel, tin, magnesium and iron.

Batteries are a store of chemical energy that is quickly converted into electrical energy when the battery is wired up. Most batteries contain reactive metals. These metals give up electrons. A simple lemon battery can be constructed using a lemon, iron nail, a copper strip, wire and a galvanometer. This setup is shown on the right.
The needle on the galvanometer is deflected when the wires are connected indicating that electrons are flowing from the iron nail to the copper.
Click to see a chemical explanation of how the battery works

By measuring the electrical current, using a galvanometer or an ammeter, we can work out the relative reactivity of metals. The greater the current produced the greater the difference in reactivity of the two metal electrodes.
For example when we have an:
- iron electrode with a copper electrode;
- zinc
electrode with a copper electrode;
- magnesium electrode with a copper electrode.
Now conduct a similar activity to see if you can place the metals copper, iron, zinc, magnesium, tin, lead and nickel, in order from lowest to highest reactivity according to your experimental results.
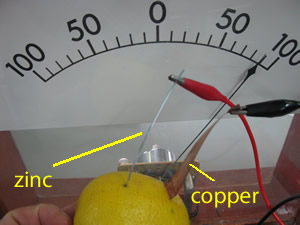
Does the current depend on the size of the electrodes used?
Consider the image on the right. It shows the current produced when a copper electrode is connected to an iron electrode. Click to see what happens when we increase the size of the copper electrode.
Design and conduct an experiment to find out if the surface area of the electrode, inserted into the lemon juice, makes a difference to the voltage or current produced. In your design take into account the following questions.
1 - What are the controlled variable/s?
- Will you change the area of one or both electrodes?
- Will you keep the metal the electrodes are made of constant
or will you change the metal?
- If you are using lemon juice, will you use the same volume of juice as you change the surface area of the electrode?
- WIll you keep the temperature of the lemon constant
2 - What is the dependent variable?
3 - What is the independent variable?
4 - What is your prediction (what do you think will happen)?
5 - How will you present your results?

| Surface area of copper electrode inserted in the juice. (cm2) | Voltage (V) |
| 2 | |
| 4 | |
| 6 | |
| 8 | |
| 10 |
Graph the results on an appropriate set of axis.
Are there any trends ? Offer an explanation.