Science of Conflict
Greek fire
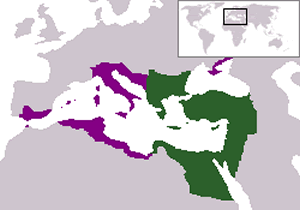
Byzantine Empire (Greek name for : king of the Romans) is the name given to the Greek-speaking Roman Empire of the Middle Ages, with Constantinople as its capital. The map on the right shows the extent of the Byzantine Empire with the green region representing the Eastern Roman Empire before the Muslim conquests. The purple region represents the greatest extent of the Byzantine Empire under the Emperor Justinian I
Greek fire was a burning liquid that was used with devastating effect in both sea and land battles. Later advances in technology saw Greek fire discharged in a burning stream from a siphon driven by a primitive pump. It was first invented in 670 by a Greek architect named Kallinikos. It is widely agreed that Greek fire was largely responsible for many Byzantine victories. As a consequence Greek fire was one of the major factors responsible for the Byzantine Empire surviving as long as it did, eventhough the number of its citizens was not enough to defend its terittories.
Greek fire was first used in 674, during the Battle of Syllaeum, to repel Muslim forces and later in 941 against Vikings.

The formula for this formidable weapon was a closely guarded secret. Even to this day, we can only speculate as to what the ingredients may have been and guess at a number of possibilities.
Suggestions include
mixtures of:
- naphtha, niter and sulfur
- petroleum, quicklime and sulfur
- phosporus and saltpeter
We are not sure if the liquid was ignited as it emerged from the siphon
or spontaneously ignited upon contact with water. If we knew with any
certainty we can have a good attempt at predicting the ingredients. For
example, if it did spontaneously combust as it touched the water then
a possible ingredient may have been calcium phosphide. Calcium phosphide
(Ca3P2) can be made by heating lime, bones and charcoal
on an open fire, and reacts with water to produce phosphine (PH3)
which ignites spontaneously.
Phosphine, also known as hydrogen phosphide, is a colourless, flammable,
extremely toxic gas, with a very strong, garlic-like odour.
Another suggestion is the reaction of quicklime with water. It is suggested that this reaction produces enough heat to ignite some mixtures of oil and saltpeter. Saltpeter (potasium nitrate KNO3) acts as an oxidant. The reaction of quicklime (calcium oxide) and water to produce slaked lime (Ca(OH)2 ) is shown below.
CaO(s) + H2O(l) => Ca(OH)2(s)
+ heat
It is worth investigating
this reaction and reaching your own conclusion as to whether it is possible
to generate enough heat to ignite a petroleum based fuel.
Click to see the
activity.
However another reaction that ignites spontaneously with water is the mixture of ammonium chloride, ammonium nitrate and zinc powder. Click to see the reaction.