Generating heat through chemical reactions

In this investigation we will see how much heat energy is generated from the reaction between aluminium metal and hydrochloric acid by measuring the change in temperature of a given mass of water.
It takes 4.2 joules of energy to raise 1 gram of water by 1 oC so if we know the mass of water and the change in temperature we can calculate the energy given out by the aluminium when it reacts with hydrochloric acid according tothe equation below.
2Al(s) + 6HCl(aq) => 3H2(g) + 2AlCl3(aq) + energy
Apparatus:
- aluminium foil
- electronic scales
- 2M HCl
- thermometer
- 100 mL measuring cylinder
- calorimeter


Now place the aluminium in the calorimeter and seal the top.
Record the temperature every 2 minutes and draw an appropriate graph to show your results.

It takes 4.2 joules of energy to raise the temperature of 1 gram of water by 1 degree Celsius.
Calculate the change in temperature of the contents placed in the calorimeter.
This will give you an indication as to how much energy was released from your mass of aluminium.
Calculations
The amount of energy released can be calculated using the formula below.
Energy (joules) = 4.2 X mass of water X change in temperature
Temperature at start ___________
Maximum temperature reached _________
Change in temperature = maximum - start = ____________
Mass of water is 80.0 g (we assume that 80.0 mL of acid solution has a mass of 80.0 grams)
What is the mass of aluminium that would deliver 6000 joules or 6 kilojoules of energy to a volume of water?
What is the maximum temperature that an 80.0 mL volume of acid solution can reach if its initial temperature is 27 oC and a 1.10 gram of aluminium foil was placed in it?
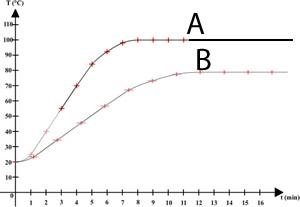
A 0.52 gram mass of aluminium foil is placed in a volume of acid. This was repeated with an identical piece of aluminium and volume of acid. The temperature for both experiments was recorded and plotted on a graph shown on the right.
Experiment A most likely had
Which of the experiments would, most likely, have unreacted aluminium present after the reaction stops.
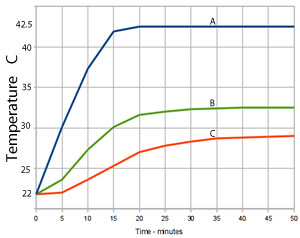
Another experiment was conducted where three different amounts of aluminium foil were added to three individual 50.0 mL volumes of HCl solution.
How much energy was given out by the aluminium foil in experiment A?
What mass of aluminium was added, assuming all the aluminium reacted?
Calculate the mass of aluminium used in experiments B and C.