1. Each of the following compounds has a molar mass of
88 g mol-1. Which one has the highest boiling point?
A. CH3CH2OCOCH3
B. CH3(CH2)2COOH
C. CH3(CH2)3CH2OН
D. CH3NH(CH2)2NHCH3
2. Which one of the following statements about cyclohexane and benzene is correct?
A. Both have structural isomers that are not cyclic.
B. Both have the same average bond strength between their carbon atoms.
C. Both are members of the same homologous series.
D. Each carbon in cyclohexane has one more valence electron than each carbon in benzene.
Solution
3. Consider the structural isomers of C5H10O that are aldehydes. Which one of the following statements is correct?
A. There are three isomers.
B. There is one isomer with an ethyl side branch.
C. There are two isomers that have one methyl side branch.
D. Each isomer has a functional group on the second carbon atom.
Solution
4. Which one of the following represents the skeletal structure for 3-chlorobutyl butanoate?
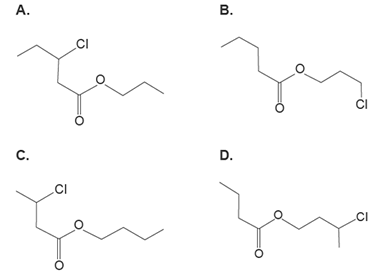
Solution
5. A triglyceride is reacted with methanol, CH3OH, in the presence of concentrated KOH(aq). The products of this reaction are glycerol and Compound J.
The molecular formula of Compound J is C19H3002. What is the molecular formula of the triglyceride?
A. C54H8203
B. C54H8206
C. C57H8403
D. C57H8606
6. When the triglyceride, in question 5, is reacted with methanol to produce glycerol and Compound J
A. H2O should be added to the KOH directly, because KOH is corrosive and causes skin burns.
B. H2O should not be added to the reaction mixture because it interferes with the desired reaction.
C. H2O should be added to separate the triglyceride from glycerol since the triglyceride is soluble in water.
D.
H2O should not be added to the reaction mixture because it increases the frequency of successful collisions.
Solution


7.
a.
How can melting point determination be used to check the components and purity of solid consumer products?
Solution
b. Linalool, C10H18O, is a colourless oil found in lavender plants. Linalool may be useful in the human body to reduce inflammation. The molar mass of linalool is 154 g moI-.
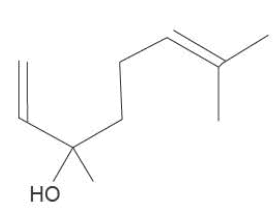
i. How many chiral centres are present in each linalool molecule?
ii. A 15.0 g mixture of linalool and hexane, C6H14 , was reacted with an excess of iodine, 12. It was found that 0.042 mol of 12 reacted with the mixture.
Calculate the percentage by mass of linalool in the original mixture.
Solution
iii. Identify two processes used to obtain purified linalool from crushed lavender
Solution
.

8. A reaction pathway begins with a hydrocarbon, Compound E.
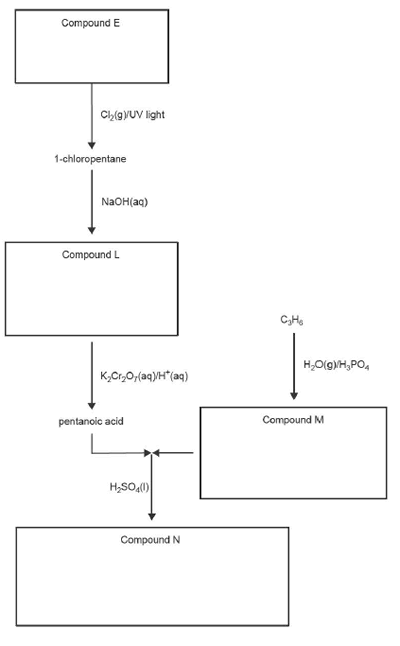
a. i. Write the molecular formula of Compound E in the box provided.
ii. State the type of reaction that produces 1-chloropentane from Compound E.
b. 1-chloropentane is reacted with NaOH to produce Compound L.
Write the semi-structural formula for Compound Lin the box provided.
c. C3H6 reacts with H2O in the presence of a H3PO4 catalyst to produce Compound M, which is a primary alcohol.
Write the IUPAC name of Compound M in the box provided.
d. Pentanoic acid is reacted with Compound M to form Compound N in the presence of concentrated sulfuric acid, H2SO4(I).
i. State a qualitative test to detect the presence of the functional group in pentanoic acid, and state the expected observation(s).
ii. Draw the skeletal structure of Compound N in the box provided.
9. Capsicum frutescens is a plant used to reduce inflammation and pain. One active ingredient of this plant
is shown below.
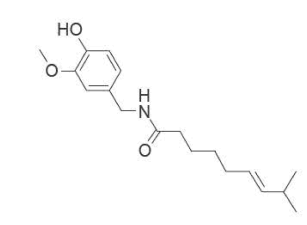
Which functional groups are present in this active ingredient?
A. alkenyl, amide, hydroxyl
B. alkenyl, amino, hydroxyl
C. amide, carboxyl, carbonyl
D. amine, carboxyl, carbonyl
Solution

10. Paracetamol, C8H9NO2, can be made using two different reactions, Reaction
P1 and Reaction P2.
Reaction P1
Liquid ethanoic anhydride, C4H603(l), is added to a mixture of solid 4-aminophenol, C6H7N0(s), and water. The resulting mixture of all reactants is heated in a hot water bath for 10 minutes.

Reaction P2
Liquid ethanoic acid, C2H4O(l), is mixed with solid 4-aminophenol, C6H7N0(s). The
reactant mixture is heated in a hot water bath for 10 minutes.

Results
• For Reaction P1 , the percentage yield is 87% and the percentage atom economy is 72%.
• For every 1.4 g of 4-aminophenol reacted in Reaction P2, 0.80 g of paracetamol
is produced.
a. i. Calculate the percentage yield and percentage atom economy of Reaction P2, and compare them to the results for Reaction P1. 4 marks
Solution
ii. Use your answer to part a.i to discuss which reaction is better aligned with green chemistry. Support your answer by referencing the relevant green chemistry principle.
Refer to item 26.ii of the Data Book. 2 marks
Solution
Prevention of waste is a green chemistry principle. Refer to item 26.ii of the Data Book. Discuss Reaction P1 and Reaction P2 in terms of this green chemistry principle. 3 marks
Solution
