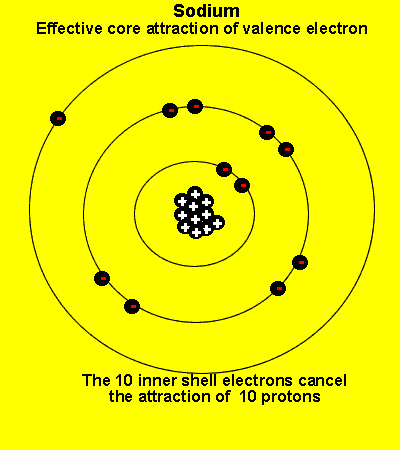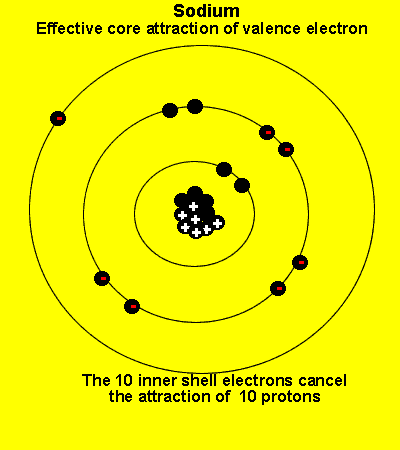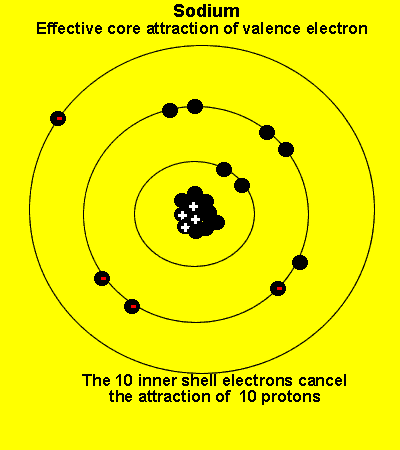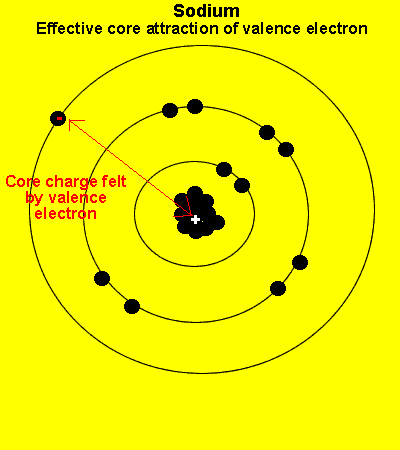
The valence electrons see a charge significantly less than that present in the nucleus. The reason is that the inner electrons cancel the effect of most of the protons.
Click to hide

The valence electrons see a charge significantly less than that present in the nucleus. The reason is that the inner electrons cancel the effect of most of the protons.
Click to hide
|
Trends in the periodic table. explained
|
|
The size of an atom is determined by the electrostatic attraction between the positive nucleus and the valence electrons. The force of this attraction is proportional to the size of charge and inversely proportional to the distance between the charges. As we go down a group the distance between the nucleus and valence electrons increases, while the charges involved stay the same. This obviously has the effect of weakening the force of attraction and the valence electron is held with less force. As a consequence, the valence electrons orbit further away from the nucleus and this gives the atom a greater size. Since the reducing strength of the atom depends on its ability to give away its valence electron it makes sense that the reducing strength of an atom increases as we move down a group. Since the force of attraction of the electron to the nucleus decreases as we move down so should the ability for the atom to give away its electron increase. The first ionisation energy and electronegativity also decrease down a group as a result of the weakening force of attraction. Across a period, the distance between the nucleus and the valence electrons remains constant but the effective core charge increases. As a result the force of attraction between the nucleus and the valence electrons increases across a period. Look at the animation above. Since the force of attraction increases across a period the trends noticed down a group are reversed. |