Covalent bonding
sharing of electrons
amongst non-metals
The simplified diagrams
below only show electrons that are being shared.
Click on the blue writing for more information
Atoms that are one or more electrons short of a stable electron configuration can share electrons amongst themselves in a process called covalent bonding.
Consider the two hydrogen atoms shown on the right. Each atom is one electron short of a stable configuration.
They can combine to share one electron each and become stable by having two electrons each in their valence shells.
The electrostatic attraction between the positive nucleus of each atom and the negative charged cloud created by the bonding electrons holds the the atoms tightly together to form molecules.
The number of covalent bonds each atom forms depends on the number of electrons it requires to achieve a stable valence shell.
Carbon forms molecules with other atoms. Carbon is four electrons short of a stable electron configuration so it will share four electrons with other non metals. Methane (CH4) is formed when carbon shares one electron with each of 4 hydrogen atoms. Each hydrogen has a stable two electrons while carbon has a stable 8 electrons in the valence shell. Water is a molecule formed between an oxygen and two hydrogen atoms. Ammonia is a molecule formed by nitrogen bonding to three hydrogens. A nitrogen atom needs three electrons to achieve a stable state, while each hydrogen needs one.
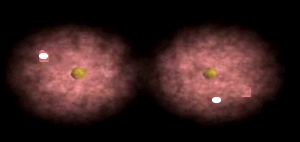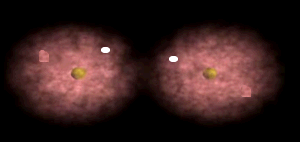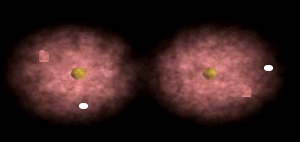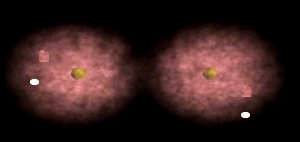
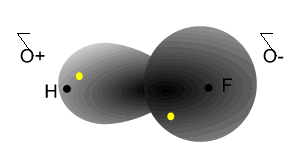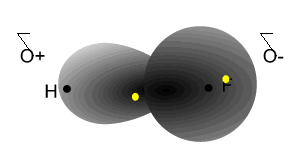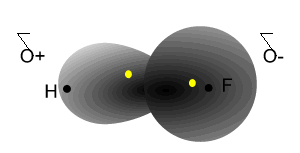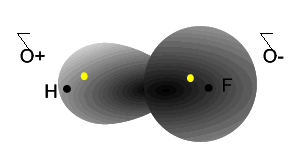
Notice how the electrons spend more time around the nucleus of fluorine.
This will be discussed in more detail later.
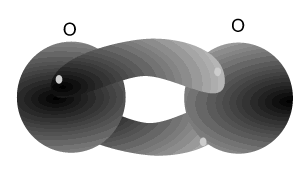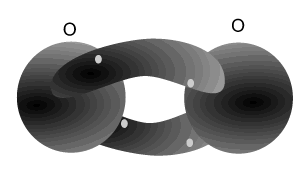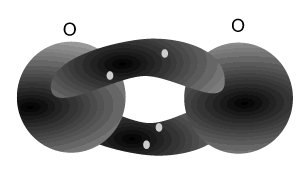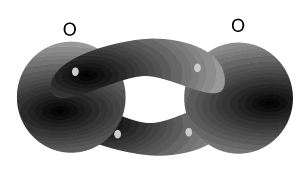
Nitrogen atoms can bond with each other to form a triple bond. A triple covalent bond comes about when both atoms share 6 electrons (3 each). Each nitrogen atom needs 3 electrons to achieve a stable (2,8) electron arrangement. So each atom shares three electrons with the other.
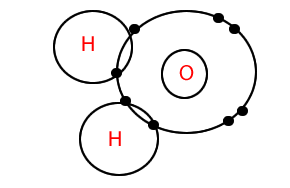
Molecules can be represented as Lewis Dot Diagrams. Consider the water molecule, shown on the right, it is represented as a Lewis dot diagram. To draw such a diagram follow two simple steps.
1) Draw each atom showing its valence electrons. Pair up the valence electrons but make sure you leave each bonding electron free. The number of bonding electrons is the number needed to reach a stable state. In this case oxygen has 6 valence electrons and needs two more electrons to become stable. Two electrons remain free on the oxygen but the others are paired.
2) Bring together the atoms so that the valence shells overlap.
Sometimes the molecucle is represented with lines. To achieve this, replace each pair of electrons with a line.
Look at the following molecules.
N2
CH4
How are the atoms held together in a covalent bond?
What determines the number of electrons each atom shares?
What type of atoms share electrons?
Draw Lewis dot diagrams of the following molecules.
SO3
NH3
CF4
CHF3
O2
Draw line diagrams of the molecules above and label the bonding and non-bonding electrons.